Pharmacodynamics is a branch of pharmacology that focuses on the study of how drugs interact with the body at the molecular, cellular, and systemic levels to produce their effects. It involves understanding the mechanisms of drug action (MOA), including drug-receptor interactions, signal transduction pathways, and the pharmacological effects of drugs.
“What drug does to the body”
Receptor Classification and Examples

Drug receptors are specialized proteins that bind to specific molecules, including endogenous substances and drugs, and elicit a biological response. There are different types of receptors, including ion channels, G-protein-coupled receptors, enzymes, and nuclear receptors.
Ion Channel Receptors/ligand-gated channels
Ion channel receptors are transmembrane proteins that form pores that allow the flow of ions across the cell membrane. Examples of ion channel receptors include voltage-gated channels, ligand-gated channels, and mechanically-gated channels.
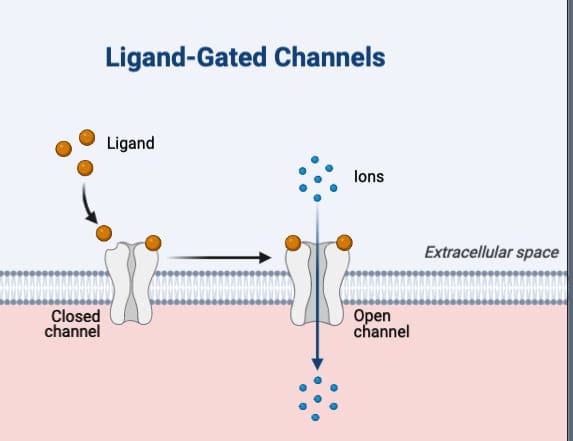
Ion channel receptors, also known as ligand-gated ion channels, are a specialized class of cell surface receptors that directly control the flow of ions across the cell membrane. These receptors are pivotal in a variety of physiological processes, including neurotransmission, muscle contraction, and sensory perception. This article delves into the structure, function, and clinical relevance of ion channel receptors.
Structural Features
Typically, ion channel receptors are pentameric proteins, meaning they are composed of five subunits. Each subunit generally has four membrane-spanning helical domains along with large intra- and extracellular segments. These subunits are usually arranged around the ion channel in a rosette-like pattern. The α subunits often contain the agonist binding sites, which are crucial for the receptor’s function.
Mechanism of Action
Upon binding of an agonist to the receptor, the ion channel opens, allowing specific ions like Na+, K+, Ca2+, or Cl- to flow through. This ion flow can lead to various cellular responses such as depolarization, hyperpolarization, or changes in the cytosolic ionic composition. Unlike G-protein coupled receptors (GPCRs), ion channel receptors do not require any coupling proteins or second messengers. The action is direct and immediate, making the onset and offset of responses extremely fast, often occurring in milliseconds.
Types of Ion Channel Receptors
Some well-known ion channel receptors include:
- Nicotinic Cholinergic Receptors: Involved in skeletal muscle contraction and neurotransmission.
- GABAA Receptors: Mediate inhibitory neurotransmission in the central nervous system.
- Glycine Receptors: Also involved in inhibitory neurotransmission, primarily in the spinal cord.
- Excitatory Amino Acid-Glutamate Receptors (Kainate, NMDA, AMPA): Play roles in synaptic plasticity and are implicated in conditions like epilepsy and Alzheimer’s disease.
- 5HT3 Receptors: Involved in serotonin signaling and are targets for antiemetic drugs.
Modulation by Secondary Ligands
Some ion channel receptors have secondary ligands that bind to allosteric sites on the receptor, modulating its activity. For example, benzodiazepines modulate the GABA-gated Cl- channels, enhancing the inhibitory effects of GABA. This makes benzodiazepines effective as anxiolytics, sedatives, and anticonvulsants.
Clinical Relevance
A large number of clinically useful drugs target ion channel receptors. For instance, benzodiazepines act on GABAA receptors, while nicotine replacement therapies target nicotinic cholinergic receptors. Understanding the mechanisms of these receptors can lead to the development of more effective and targeted therapies.
Ion channel receptors are integral components of cellular signalling pathways, directly controlling ion flow and influencing many physiological processes. Their rapid action and direct mechanism make them unique among cell surface receptors. Understanding their structure and function is not only scientifically intriguing but also clinically vital for the development of new therapeutic agents.
G-protein-coupled receptors (GPCRs)
G-protein-coupled receptors (GPCRs) are the largest class of receptors that span the cell membrane seven times. GPCRs bind to extracellular ligands, which activate intracellular signalling pathways that lead to a biological response. Examples of GPCRs include the beta-adrenergic receptor, opioid receptor, and histamine receptor.
G-protein coupled Receptors (GPCRs) are a large family of cell membrane receptors that play a critical role in cellular signaling. These receptors are involved in a wide range of physiological processes, from sensory perception to immune responses. This article aims to provide an in-depth understanding of the structure, function, and signaling pathways of GPCRs.
Structural Organization
GPCRs share a common structural organization, featuring seven α-helical membrane-spanning hydrophobic amino acid segments. These segments are connected by three extracellular and three intracellular loops. The agonist binding site is located between the helices on the extracellular face, while another recognition site on the cytosolic segments binds the coupling G-protein.
G-Proteins: The Mediators
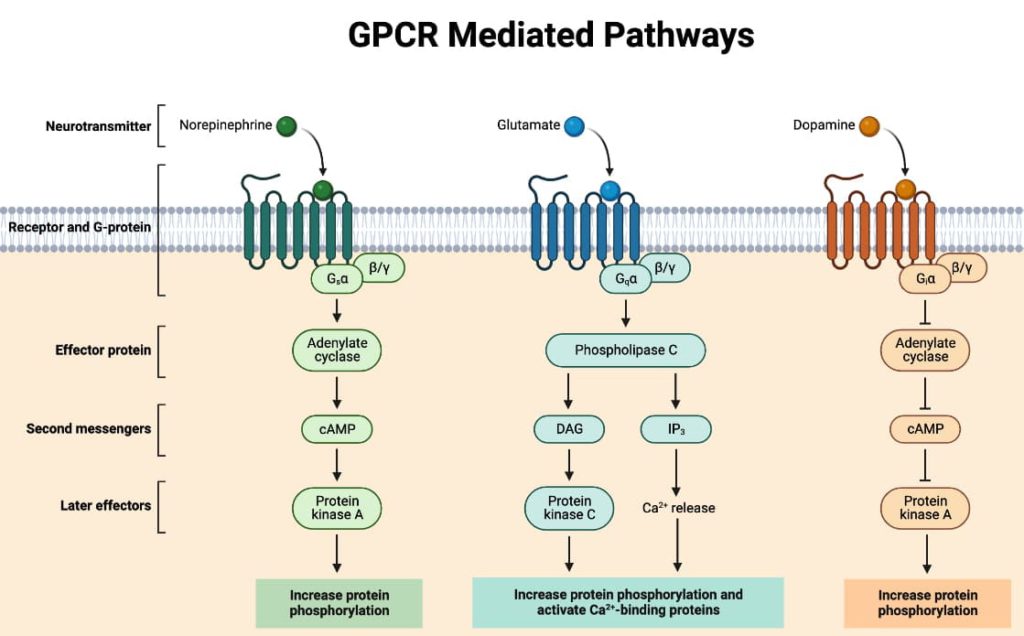
G-proteins are heterotrimeric in composition, consisting of α, β, and γ subunits. In their inactive state, GDP is bound to the α subunit. Activation through the receptor leads to the displacement of GDP by GTP, causing the α-subunit to dissociate from the βγ dimer. This activated α-subunit then either activates or inhibits the effector molecule. The βγ dimer also has roles, such as activating receptor-operated K+ channels and inhibiting voltage-gated Ca2+ channels.
Types of G-Proteins
- Gs: Activates adenylyl cyclase, opens Ca2+ channels
- Gi: Inhibits adenylyl cyclase, opens K+ channels
- Go: Inhibits Ca2+ channels
- Gq: Activates phospholipase C
Receptor Coupling
A single receptor can utilize more than one G-protein, a phenomenon known as agonist pleotropy. For example, the muscarinic M1 and M3 receptors are associated with Gq, while the muscarinic M2 receptor is associated with Gi and Go. This allows for a diverse range of physiological responses.
Major Effector Pathways
1. Adenylyl Cyclase: cAMP Pathway
Activation of adenylyl cyclase leads to the accumulation of cAMP, which acts mainly through cAMP-dependent protein kinase (PKA). This pathway regulates a variety of cellular functions, including muscle contraction, hormone synthesis, and ion channel regulation.
2. Phospholipase C: IP3-DAG Pathway
Activation of phospholipase C by Gq leads to the generation of second messengers IP3 and DAG. IP3 mobilizes Ca2+ from endoplasmic reticular depots, while DAG activates protein kinase C (PKC). This pathway regulates a wide range of physiological responses, including muscle contraction and cell proliferation.
3. Channel Regulation
G-proteins can directly regulate ion channels specific for Ca2+ and K+. For example, Gs opens Ca2+ channels in the myocardium and skeletal muscles, while Gi and Go open K+ channels in the heart and smooth muscle.
Regulation and Termination
The α-subunit has intrinsic GTPase activity, allowing it to hydrolyze GTP to GDP and rejoin its other subunits. This process is regulated by a protein called ‘regulator of G protein signaling’ (RGS). The signaling is terminated by the degradation of second messengers like cAMP and IP3.
GPCRs are incredibly versatile and complex signaling molecules that are crucial for a wide range of physiological processes. Understanding their structure and function is essential for the development of targeted therapies for a variety of diseases.
Receptor tyrosine kinases (RTKs)/Enzyme-linked Receptors
Enzyme receptors are proteins that possess enzymatic activity, such as protein kinases, which phosphorylate specific proteins, leading to a biological response.
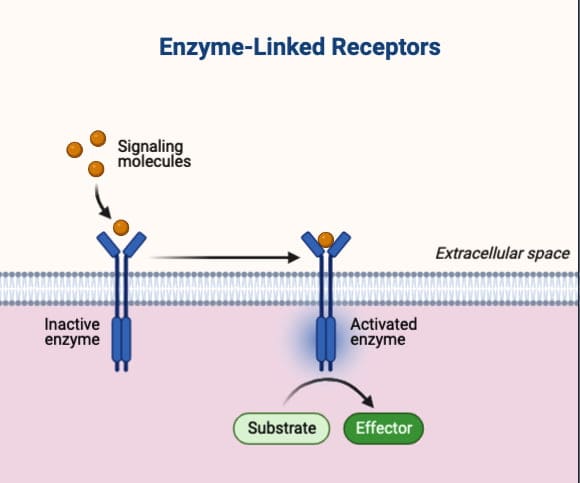
Transmembrane enzyme-linked receptors are a distinct class of cell surface receptors that are primarily utilized by peptide hormones. These receptors play a crucial role in regulating a wide array of cellular functions, including metabolic reactions, cell growth, and differentiation. This article aims to provide an in-depth understanding of the structure, function, and clinical relevance of these receptors.
Structural Overview
Transmembrane enzyme-linked receptors typically consist of a large extracellular ligand-binding domain connected to an intracellular enzymatic subunit via a single transmembrane helical peptide chain. The intracellular enzyme is often a protein kinase, although in some cases, it can be guanylyl cyclase.
Types of Protein Kinases
- Receptor Tyrosine Kinases (RTKs): These kinases phosphorylate tyrosine residues on substrate proteins. Examples include insulin, epidermal growth factor (EGF), and nerve growth factor (NGF) receptors.
- Serine/Threonine Kinases: These kinases phosphorylate serine or threonine residues on target proteins, as seen in transforming growth factor (TGF) receptors.
Mechanism of Action
In the unliganded state, the receptor exists as an inactive monomer. Hormone binding induces receptor dimerization and conformational changes, activating the kinase to autophosphorylate tyrosine residues. This increases their affinity for substrate proteins containing SH2 domains, which are then phosphorylated and released to propagate the signaling cascade.
Role of SH2 Domains
A variety of intracellular signaling proteins have SH2 domains. These domains enable the receptor to control the phosphorylation of key enzymes, ion channels, and transporters, thereby regulating diverse cellular functions.
Second Messengers
Some transmembrane enzyme-linked receptors activate phospholipase Cγ (PLCγ), generating inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG) as second messengers.
Time Frame of Action
The onset of responses through these receptors can range from a few minutes to a few hours, making them relatively fast-acting compared to other receptor types.
Regulation and Downregulation
Receptor dimerization often leads to internalization and degradation of the receptor, serving as a mechanism for downregulation. Mutations that interfere with this process can contribute to the development of certain malignancies.
Clinical Relevance
Inhibitors targeting specific RTKs involved in growth factor signaling represent a new class of targeted anticancer drugs. Additionally, these receptors are implicated in various other diseases and are, therefore, important targets for therapeutic intervention.
Special Cases: Guanylyl Cyclase
In some instances, the intracellular enzyme is guanylyl cyclase, as seen in atrial natriuretic peptide (ANP) receptors. Activation of these receptors generates cyclic GMP (cGMP) as a second messenger, which in turn activates cGMP-dependent protein kinase (PKG) to modulate cellular activity.
Transmembrane enzyme-linked receptors are versatile and complex molecules that play a pivotal role in cellular signaling. Understanding their structure and function is essential for the development of targeted therapies for a range of diseases, including cancer.
Nuclear Receptors/intracellular receptors
Nuclear receptors are intracellular receptors that bind to hormones and other molecules and regulate gene expression. Examples of nuclear receptors include the estrogen receptor, androgen receptor, and glucocorticoid receptor.
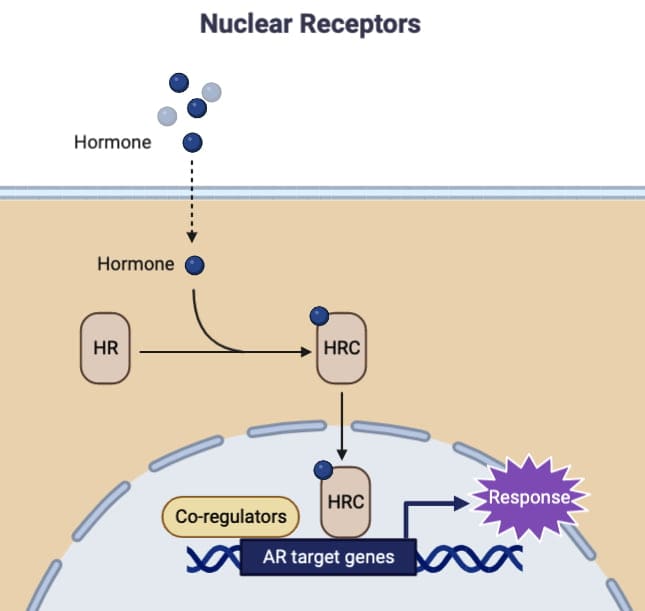
Unlike membrane-bound receptors that mediate rapid cellular responses, some receptors function within the cell to regulate gene expression. These intracellular receptors, including transcription factors and nuclear receptors, respond to lipid-soluble messengers that can penetrate the cell membrane. This article explores the structure, function, and biological implications of these unique receptors.
Structural Features
Intracellular receptors are soluble proteins located either in the cytoplasm or the nucleus. These receptors are usually bound to heat shock proteins (HSP-90) and possibly other proteins that prevent them from binding to DNA. The receptor has different domains, including a hormone-binding domain near the carboxy terminus and a DNA-binding domain typically located in the middle of the molecule.
Mechanism of Action
When a hormone binds to the receptor, the attached proteins like HSP-90 are released. This allows the receptor to dimerize and adopt a conformation that enables it to bind to DNA. The ligand-bound receptor dimer moves to the nucleus, where it associates with co-activators or co-repressors that modulate its activity. The receptor complex then binds to specific DNA sequences, known as hormone response elements, to either facilitate or repress the expression of target genes.
Specificity
Different hormones bind to their specific receptors and direct unique patterns of gene expression. The specificity is determined by the hormone-binding domain and the DNA-binding domain of the receptor. For example, glucocorticoids, mineralocorticoids, androgens, estrogens, and progesterone each have their own receptors and influence distinct sets of genes.
Ligand-Specific Conformations
Different ligands can induce unique conformations in the same receptor, allowing for tissue-specific actions. For instance, selective estrogen receptor modulators (SERMs) like tamoxifen and raloxifene have different effects on various estrogenic target tissues.
Time Course and Duration of Action
This mechanism of action is relatively slow, taking hours to manifest, as it involves the synthesis of new proteins. However, the effects are often long-lasting, as many of the proteins produced have slow turnover rates and persist even after the hormone has been eliminated.
Clinical Implications
Understanding these receptors is crucial for pharmacological interventions. For example, SERMs are used in breast cancer treatment and osteoporosis prevention. Moreover, mutations in these receptors or their regulatory proteins can lead to various diseases, including hormone-dependent cancers.
Intracellular receptors that regulate gene expression offer a unique mechanism for cellular control. They are pivotal in mediating the long-term effects of hormones and other lipid-soluble messengers. Understanding their function and regulation provides valuable insights into cellular physiology and offers avenues for targeted therapeutic strategies.
Signal Transduction Mechanisms
Signal transduction mechanisms are the series of biochemical steps that occur when a cell receives a signal. These mechanisms allow cells to communicate with each other and respond to various stimuli.
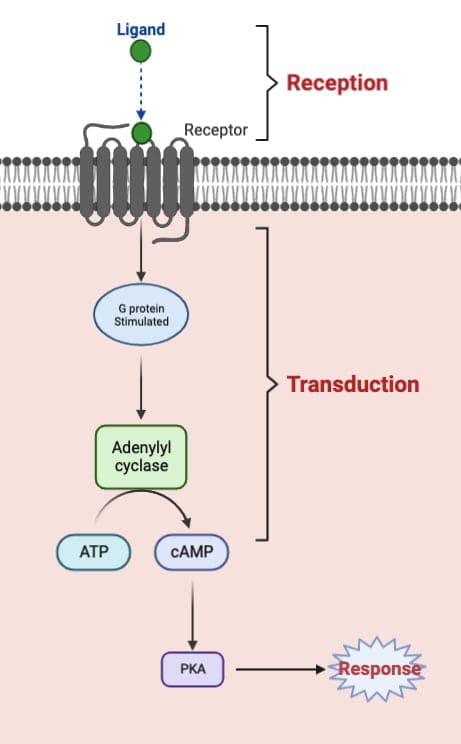
The basic steps of signal transduction are:
- Reception: The signaling molecule, also known as a ligand, binds to a receptor.
- Transduction: The receptor undergoes a conformational change, which activates intracellular signaling pathways. These pathways can involve second messengers, such as cyclic AMP (cAMP), cyclic GMP (cGMP), diacylglycerol (DAG), calcium ion (Ca2+), and inositol triphosphate (IP3).
- Response: The final step of signal transduction is the cellular response. This can involve changes in gene expression, protein synthesis, enzyme activity, ion channels, and membrane transport.
There are several types of signal transduction mechanisms, including:
- G protein-coupled receptors (GPCRs): These receptors are the largest family of cell surface receptors and are involved in a wide variety of physiological processes, such as vision, smell, and neurotransmission.
- Receptor tyrosine kinases (RTKs)/enzyme-linked receptors: These receptors are involved in the regulation of cell growth, differentiation, and survival.
- Ion channel receptors: These receptors are involved in the regulation of ion flux across the cell membrane and are involved in processes such as neurotransmission and muscle contraction.
- Intracellular receptors: These receptors are located inside the cell and are activated by ligands that can cross the cell membrane, such as hormones.
Signal transduction mechanisms are essential for the normal functioning of cells and organisms. Disruptions in these mechanisms can lead to diseases such as cancer, diabetes, and Alzheimer’s disease. Understanding these mechanisms is, therefore, crucial for developing new therapies and treatments for these conditions.
Dose-response relationships
Drug Selectivity, Safety, and Risk-Benefit Ratio
Drug Specificity: A Key Factor in Pharmacological Actions
Drug-Receptor Interactions: Agonists and Antagonists
Factors Influencing Drug Action
Several factors, including genetics, age, gender, and environmental factors, influence drug response. Genetic differences can affect drug metabolism, receptor expression, and drug sensitivity. Age and gender can also influence drug response due to differences in body composition and hormonal factors. Environmental factors such as diet and exposure to toxins can also affect drug response.
Quiz on Pharmacokinetics and Pharmacodynamics
📚 AI Pharma Quiz Generator
🎉 Quiz Results
Medical Disclaimer
The medical information on this post is for general educational purposes only and is provided by Pharmacology Mentor. While we strive to keep content current and accurate, Pharmacology Mentor makes no representations or warranties, express or implied, regarding the completeness, accuracy, reliability, suitability, or availability of the post, the website, or any information, products, services, or related graphics for any purpose. This content is not a substitute for professional medical advice, diagnosis, or treatment; always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition and never disregard or delay seeking professional advice because of something you have read here. Reliance on any information provided is solely at your own risk.