Introduction
Overview of Estrogens: Definition and Biological Significance
Estrogens are a group of steroid hormones crucial for the development and functioning of both female and male reproductive systems, although they are primarily considered female hormones. They play a significant role in sexual and reproductive development, particularly in women. Beyond their reproductive functions, estrogens are also involved in bone health, cardiovascular function, skin repair, brain health, and the regulation of cholesterol levels.
Historical Perspective of Estrogen Research and Use
The study and application of estrogens have evolved significantly over the years. Initially, estrogen research was focused on understanding its role in female reproduction. The discovery and isolation of estrogen in the early 20th century led to the development of synthetic estrogens and estrogen-based therapies, such as hormone replacement therapy (HRT) and oral contraceptives. Over the years, research has expanded to include the effects of estrogen on various diseases, such as osteoporosis and breast cancer.
Basic Biochemistry of Estrogens
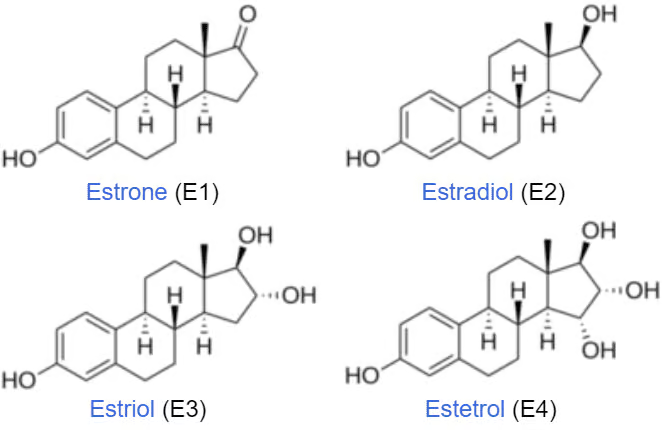
Estrogens are derived from cholesterol and are part of the steroid hormone family. The primary naturally occurring estrogens in women are estrone (E1), estradiol (E2), estriol (E3) and estetrol (E4). Estradiol is the most potent and prevalent form. These hormones are synthesized mainly in the ovaries of premenopausal women and, to a lesser extent, in adipose tissue and the adrenal glands. Estetrol is the native estrogen of fetal life and is produced exclusively by the fetal liver. In men, small amounts of estrogen are produced in the testes and adrenal glands.
Sources of Estrogens in the Body
In women, the primary source of estrogen is the ovaries, particularly during the reproductive years. After menopause, estrogen production significantly decreases, with most of the residual estrogen being produced in peripheral tissues. In men, estrogens are produced in small quantities by the testes and adrenal glands. Additionally, in both sexes, peripheral conversion of androgens (male hormones) into estrogens occurs.
Role of Estrogen in Different Physiological Processes
Estrogens influence a wide range of physiological processes. In women, they regulate the menstrual cycle, support pregnancy, and maintain secondary sexual characteristics. In both sexes, estrogens contribute to bone density and strength, influence lipid metabolism, affect skin and hair health, modulate mood and cognitive functions, and play a role in cardiovascular health.
Estrogen Receptors
Structure and Function of Estrogen Receptors (ERs)
Estrogen receptors are a group of proteins found inside and on cells. They are part of the nuclear receptor family and are primarily responsible for mediating the effects of estrogen. When estrogen binds to these receptors, it can directly influence the expression of specific genes (genomic action) or interact with other signalling pathways (non-genomic action).
Types of ERs: ERα and ERβ
There are two main types of estrogen receptors: ERα and ERβ. These receptors have different distributions in the body and can have different effects when activated. ERα is predominantly found in the uterus, liver, and mammary glands, whereas ERβ is found in the ovaries, lungs, gastrointestinal tract, and central nervous system.
Mechanism of Action: Genomic and Non-Genomic Pathways
The genomic pathway involves estrogen binding to its receptor, which then binds to specific DNA sequences, leading to changes in gene transcription. This process is relatively slow, taking hours or days. The non-genomic pathway involves the rapid activation of signalling cascades and does not directly affect gene transcription. This pathway can lead to quick cellular responses.
ER Distribution in Various Tissues and Its Implications
The distribution of ERs in different tissues underlines the varied effects of estrogens. For instance, the presence of ERα in the uterus and mammary glands explains the role of estrogen in reproductive tissues. The presence of ERβ in the brain and cardiovascular system suggests a role in cognitive function and cardiovascular health.
Interactions with Other Receptor Systems
Estrogen receptors can interact with other hormone receptors and signalling pathways, leading to a complex network of hormonal regulation. For example, in breast tissue, estrogen receptors interact with progesterone receptors, and in bone, they interact with androgen receptors. These interactions are crucial for the coordinated regulation of physiological processes.
This introduction provides a foundational understanding of the biochemistry, sources, roles, and receptor mechanisms of estrogen, setting the stage for a deeper exploration of its pharmacological aspects in subsequent sections.
Agonists
Definition and Mechanism of Action
Estrogen agonists are compounds that bind to and activate estrogen receptors (ERs), mimicking the effects of natural estrogens in the body. They bind to ERα and/or ERβ, triggering a series of intracellular events that culminate in gene expression changes and physiological effects. The mechanism of action can involve both genomic pathways, which affect gene transcription, and non-genomic pathways, which lead to rapid cellular responses.
Natural and Synthetic Estrogen Agonists
Natural estrogen agonists include endogenous estrogens like estradiol, estrone, and estriol. Synthetic agonists, designed to mimic the action of natural estrogens, include compounds such as ethinylestradiol (used in oral contraceptives) and conjugated estrogens (used in hormone replacement therapy). These synthetic variants often have higher potency or stability than natural estrogens.
Therapeutic Uses of Estrogen Agonists
Estrogen agonists are used in various therapeutic contexts:
- Hormone Replacement Therapy (HRT): To alleviate menopausal symptoms and prevent osteoporosis.
- Contraception: as a component of combined oral contraceptive pills.
- Hypogonadism, ovarian failure, and other hormonal imbalances: to supplement estrogen.
- Certain skin conditions: such as acne or dermatitis.
- Breast and Prostate Cancer: Certain types of cancers respond to estrogen modulation.
Pharmacokinetics and Pharmacodynamics
The pharmacokinetics of estrogen agonists vary depending on their formulation. Oral estrogens are metabolized in the liver, which can lead to a first-pass effect. Transdermal preparations bypass the liver, reducing this effect. Pharmacodynamically, these agents bind to estrogen receptors, leading to variable activation depending on the tissue distribution of the receptors and the specific pharmacologic properties of the agonist.
Adverse Effects and Contraindications
Common adverse effects include nausea, breast tenderness, and headaches. More serious risks involve thromboembolism, stroke, and, with prolonged use, an increased risk of certain cancers like breast cancer. Contraindications include a history of hormone-sensitive cancers, thromboembolic disorders, and undiagnosed vaginal bleeding.
Antagonists
Definition and Mechanism of Action
Estrogen antagonists are compounds that bind to estrogen receptors but do not activate them, blocking the action of endogenous estrogens. They can be classified as pure antagonists, which completely block estrogen effects, or partial antagonists, which may have agonist activity in certain tissues.
Types of Estrogen Antagonists: Pure Antagonists and Partial Antagonists
Pure antagonists, like fulvestrant, bind and degrade estrogen receptors, leading to a decrease in receptor levels. Partial antagonists, like tamoxifen, have mixed agonist/antagonist activity; they act as antagonists in some tissues (like breast tissue) and agonists in others (like bone and uterine tissue).
Clinical Applications: From Oncology to Gynecology
- Breast Cancer: Tamoxifen and fulvestrant are used in estrogen receptor-positive breast cancer.
- Gynecological Conditions: Such as endometriosis and uterine fibroids.
- Infertility Treatment: To manipulate the reproductive cycle through assisted reproduction techniques.
Pharmacokinetics of Estrogen Antagonists
The pharmacokinetics of estrogen antagonists can vary. For example, tamoxifen is orally administered and metabolized in the liver into active metabolites. Fulvestrant is administered via injection and has a different metabolic pathway. The elimination half-life, bioavailability, and interactions with other drugs can vary significantly among different antagonists.
Adverse Effects and Safety Profile
Side effects of estrogen antagonists can include hot flashes, mood swings, vaginal dryness, and, in the case of tamoxifen, an increased risk of uterine cancer and thromboembolic events. Fulvestrant can cause injection site reactions and gastrointestinal symptoms. Monitoring and managing these side effects is crucial in long-term therapies.
Selective Estrogen Receptor Modulators (SERMs)
Definition and Mechanism of Action
Selective Estrogen Receptor Modulators (SERMs) are a class of drugs that act on the estrogen receptors (ER) with a selective action, either as agonists or antagonists, depending on the target tissue. They bind to estrogen receptors and can elicit variable responses in different tissues. This selectivity allows SERMs to confer the beneficial effects of estrogen in certain tissues while avoiding or counteracting its potential adverse effects in others.
Differentiating SERMs from Agonists and Antagonists
Unlike estrogen agonists that uniformly stimulate estrogen receptors or antagonists that consistently block them, SERMs can selectively block or activate estrogen receptors depending on the tissue type. For example, they may act as antagonists in breast tissue but as agonists in bone and cardiovascular systems.
Therapeutic Uses of SERMs: Osteoporosis, Breast Cancer, etc.
- Breast Cancer: SERMs like tamoxifen are used in the treatment and prevention of hormone-receptor-positive breast cancer.
- Osteoporosis: Drugs like raloxifene are used to prevent and treat osteoporosis in postmenopausal women by mimicking estrogen’s bone-preserving action.
- Reproductive Health: Some SERMs can be used in fertility treatments due to their effect on ovulation.
- Prevention of Cardiovascular Disease: SERMs may reduce the risk of cardiovascular diseases in postmenopausal women by favourably influencing lipid profiles.
Examples: Tamoxifen, Raloxifene, and Others
- Tamoxifen: Primarily used for breast cancer treatment and prevention, acting as an antagonist in breast tissue.
- Raloxifene: Used in the prevention and treatment of osteoporosis, acting as an agonist in bone tissue while being an antagonist in breast and uterine tissue.
- Others: Clomiphene, used in ovulation induction, and newer SERMs being developed for various indications.
Side Effects and Management
Common side effects of SERMs include hot flashes, leg cramps, and an increased risk of venous thromboembolism. Tamoxifen is associated with an increased risk of endometrial cancer. Management of these side effects involves regular monitoring, patient education, and symptomatic treatment. The choice of a specific SERM and the decision to continue treatment should be based on a thorough evaluation of the benefits and risks for the individual patient.
Estrogen and Menopause
Role of Estrogen in Menopause and Post-Menopausal Health
During menopause, the decline in estrogen levels can lead to various symptoms like hot flashes, night sweats, mood changes, and vaginal dryness. Long-term estrogen deficiency is associated with osteoporosis, skin changes, and an increased risk of cardiovascular disease. Estrogen plays a crucial role in managing these post-menopausal changes and symptoms.
Hormone Replacement Therapy (HRT): Benefits and Risks
HRT involves the administration of estrogen, often in combination with progesterone, to alleviate menopausal symptoms and prevent the long-term consequences of estrogen deficiency. While HRT can significantly improve quality of life and reduce the risk of osteoporosis and possibly cardiovascular disease, it is associated with risks such as thromboembolic events, stroke, and, in certain formulations, an increased risk of breast cancer.
Non-Hormonal Alternatives for Menopause Management
For women who cannot or choose not to use HRT, non-hormonal alternatives include lifestyle modifications, dietary changes, and medications such as selective serotonin reuptake inhibitors (SSRIs) for mood swings and hot flashes and bisphosphonates for osteoporosis prevention.
Current Guidelines and Recommendations
Current guidelines recommend individualized decision-making for HRT, considering the severity of menopausal symptoms, the woman’s health history, and her risk profile for breast cancer, cardiovascular disease, and osteoporosis. HRT is generally recommended to be used at the lowest effective dose for the shortest duration possible. Regular monitoring and re-evaluation are essential components of HRT management.
Estrogen in Male Health
Role of Estrogen in Male Physiology
While often associated with female health, estrogen plays a significant role in male physiology as well. In men, estrogen is mainly produced by the aromatization of testosterone and is crucial for regulating libido, erectile function, and spermatogenesis. It also contributes to bone density maintenance, brain function, and cardiovascular health. Estrogen helps in modulating lipid metabolism and can influence mood and emotional states in men.
Estrogen Imbalance in Men: Causes and Consequences
An imbalance in estrogen levels in men can lead to various health issues. Elevated estrogen levels, a condition known as estrogen dominance, can result from obesity, excessive alcohol consumption, or certain medications. It can lead to symptoms like gynecomastia (development of breast tissue), infertility, erectile dysfunction, and loss of muscle mass. Low levels of estrogen can lead to osteoporosis, increased fat accumulation, and potential cardiovascular risks.
Therapeutic Approaches to Manage Estrogen Levels in Men
Treatment strategies depend on the underlying cause of the imbalance. In cases of high estrogen levels, lifestyle modifications such as weight loss and reducing alcohol intake can be helpful. Medications like aromatase inhibitors, which prevent the conversion of testosterone to estrogen, may be prescribed. For low estrogen levels, the approach might involve treating the underlying condition affecting testosterone levels, as this indirectly impacts estrogen levels.
Estrogen in Contraception
Estrogen Components in Oral Contraceptive Pills
Estrogen, often in the form of ethinylestradiol, is a key component in many combined oral contraceptive pills (OCPs). It works in conjunction with a progestin component to prevent pregnancy. The estrogen in OCPs primarily functions to inhibit ovulation by suppressing the release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from the pituitary gland.
Mechanism of Action in Contraception
The primary mechanism of action of estrogen in contraception is the suppression of ovulation. By inhibiting FSH and LH, estrogen prevents the maturation and release of the ovum. Additionally, estrogen contributes to contraceptive efficacy by stabilizing the endometrial lining, reducing the likelihood of breakthrough bleeding, and making the cervical mucus less permeable to sperm.
Benefits, Risks, and Selection Criteria for Estrogen-containing Contraceptives
Benefits of estrogen-containing contraceptives include highly effective pregnancy prevention, regulation of menstrual cycles, reduction of menstrual cramps, and alleviation of acne. However, risks include an increased chance of thromboembolic events (blood clots), especially in women who smoke or are over 35 years old. When selecting an estrogen-containing contraceptive, factors like the individual’s health history, age, smoking status, and risk factors for cardiovascular disease are considered.
Non-contraceptive Benefits and Risks
Beyond contraception, these pills offer benefits like reduced risk of ovarian and endometrial cancers, improved menstrual regularity, and management of conditions like polycystic ovary syndrome (PCOS) and endometriosis. However, risks involve a slightly increased risk of breast and cervical cancer with long-term use, as well as potential effects on mood and libido. It’s crucial for healthcare providers to weigh these benefits and risks when prescribing estrogen-containing contraceptives.
Drug Interactions and Contraindications
Interaction of Estrogens with Other Drugs
Estrogens can interact with various medications, which can alter their efficacy or increase the risk of adverse effects. Notable interactions include:
- Inducers of Cytochrome P450 Enzymes: Medications like rifampin, phenytoin, and certain barbiturates can increase the metabolism of estrogens, reducing their effectiveness.
- Corticosteroids: Estrogens can potentiate the effects of corticosteroids, necessitating dosage adjustments.
- Anticoagulants: Estrogens can alter the anticoagulant effect of warfarin and similar drugs, requiring careful monitoring.
- Thyroid Hormone Replacement: Estrogens can increase thyroid-binding globulin levels, affecting thyroid hormone levels in the body.
Contraindications of Estrogen Therapy
Estrogen therapy is contraindicated in certain conditions, including:
- History of Estrogen-Sensitive Cancers: Such as breast or uterine cancer.
- Thromboembolic Disorders: Due to the increased risk of blood clots.
- Liver Disease: Estrogens can exacerbate liver disease.
- Pregnancy: Estrogens are contraindicated due to potential harm to the fetus.
- Undiagnosed Vaginal Bleeding: Requires diagnosis before initiation of estrogen therapy.
Monitoring and Managing Drug Interactions
Regular monitoring and review are essential when patients are on estrogen therapy, especially if they are taking other medications. It involves:
- Regular Blood Tests: To monitor hormone levels, liver function, and blood clotting parameters.
- Clinical Evaluation: Assessing for side effects or symptoms that might indicate adverse interactions.
- Adjusting Dosages: Of either estrogen or the interacting drug, as necessary.
Future Directions
Research Trends in Estrogen Pharmacology
Current research in estrogen pharmacology is exploring:
- Improved SERMs and SARMs (Selective Androgen Receptor Modulators): To maximize therapeutic benefits while minimizing side effects.
- Role of Estrogen in Neurodegenerative Diseases: Investigating its potential in conditions like Alzheimer’s disease.
- Estrogen in Cardiovascular Health: Understanding how estrogen influences heart disease, especially in postmenopausal women.
Emerging Therapies and Potential Uses of Estrogens
Emerging therapies include:
- Targeted Estrogen Therapy: Developing formulations that target specific tissues to reduce systemic side effects.
- Use in Male Health: Investigating the role of estrogen in treating conditions like prostate cancer and osteoporosis in men.
- Novel Drug Delivery Systems: Such as transdermal or localized delivery methods for more controlled dosing.
Genetic and Personalized Medicine Approaches
The future of estrogen therapy lies in personalized medicine, which considers an individual’s genetic makeup in therapy planning. This approach includes:
- Genetic Testing: To predict response to estrogen therapy and assess risk for side effects, particularly in cancer treatment.
- Tailored Therapies: Based on individual hormonal profiles, genetic predispositions, and specific health conditions.
- Research on Genetic Variants: Understanding how variations in estrogen receptor genes affect disease risk and treatment response.
The field of estrogen pharmacology is evolving rapidly, with ongoing research aimed at maximizing therapeutic benefits while minimizing risks and adverse effects. Personalized medicine approaches are expected to play a significant role in the future of estrogen therapy.
Conclusion
Summary of Key Points
The pharmacology of estrogen encompasses a broad range of topics, from the basic biochemistry and physiological roles of estrogens to the therapeutic applications and potential risks associated with estrogen therapy. Key points include:
- Estrogens’ Role: Estrogens play vital roles in both female and male physiology, influencing reproductive health, bone density, cardiovascular function, and more.
- Therapeutic Uses: Estrogen therapy is used in various contexts, such as menopause management, contraception, and the treatment of certain cancers.
- SERMs and Antagonists: Selective Estrogen Receptor Modulators (SERMs) and estrogen antagonists offer targeted approaches to therapy, balancing estrogen’s beneficial effects while minimizing potential risks.
- Drug Interactions and Safety: The use of estrogen therapy requires careful consideration of potential drug interactions and contraindications to ensure patient safety.
Balancing Efficacy and Safety in Estrogen Therapy
The challenge in estrogen therapy lies in maximizing efficacy while minimizing risks. This balance is achieved through:
- Personalized Approach: Tailoring treatments based on individual patient profiles, including age, health status, and genetic makeup.
- Monitoring and Adjustment: Regular monitoring of therapy effects and adjusting dosages or treatment strategies as needed.
- Awareness of Risks: Educating patients about potential side effects and ensuring informed decision-making.
The Future of Estrogen Pharmacology in Medicine
Looking ahead, the field of estrogen pharmacology is poised for significant advancements:
- Innovations in Treatment: Ongoing research is likely to yield new treatments that offer greater efficacy and safety, including personalized medicine approaches.
- Broader Therapeutic Applications: Expanding the use of estrogens and SERMs in areas like neurodegenerative diseases and male health.
- Technological Advancements: Utilizing technology for better drug delivery systems and more precise targeting of estrogen therapy.
In conclusion, estrogen pharmacology is a dynamic and critical area of medicine with far-reaching implications for health and disease management. Continued research and innovation are essential for advancing our understanding and application of estrogen therapy, ultimately improving patient outcomes and quality of life.