Introduction
Type 2 Diabetes Mellitus (T2DM) has become a global health challenge, characterized by insulin resistance and a relative deficiency of insulin secretion, resulting in persistent hyperglycemia. Over time, poorly controlled T2DM can lead to complications affecting the cardiovascular system, kidneys, eyes, and nervous system. Effective pharmacotherapy for T2DM aims to enhance glycemic control, prevent disease progression, reduce the risk of cardiovascular events, and improve patient quality of life.
The evolution of antidiabetic drugs in recent decades has significantly expanded the therapeutic armamentarium, enabling more personalized and safe treatments to address the heterogeneous presentation of T2DM. This article presents a thorough exploration of pharmacotherapy for type 2 diabetes, detailing the primary drug classes, mechanism of action, clinical efficacy, side effects, and roles in treatment algorithms. It also highlights the importance of lifestyle interventions and patient-centered care. References are drawn from “Goodman & Gilman’s The Pharmacological Basis of Therapeutics” (13th Edition), “Katzung BG, Basic & Clinical Pharmacology” (15th Edition), and “Rang & Dale’s Pharmacology” (8th Edition).
Pathophysiology of Type 2 Diabetes
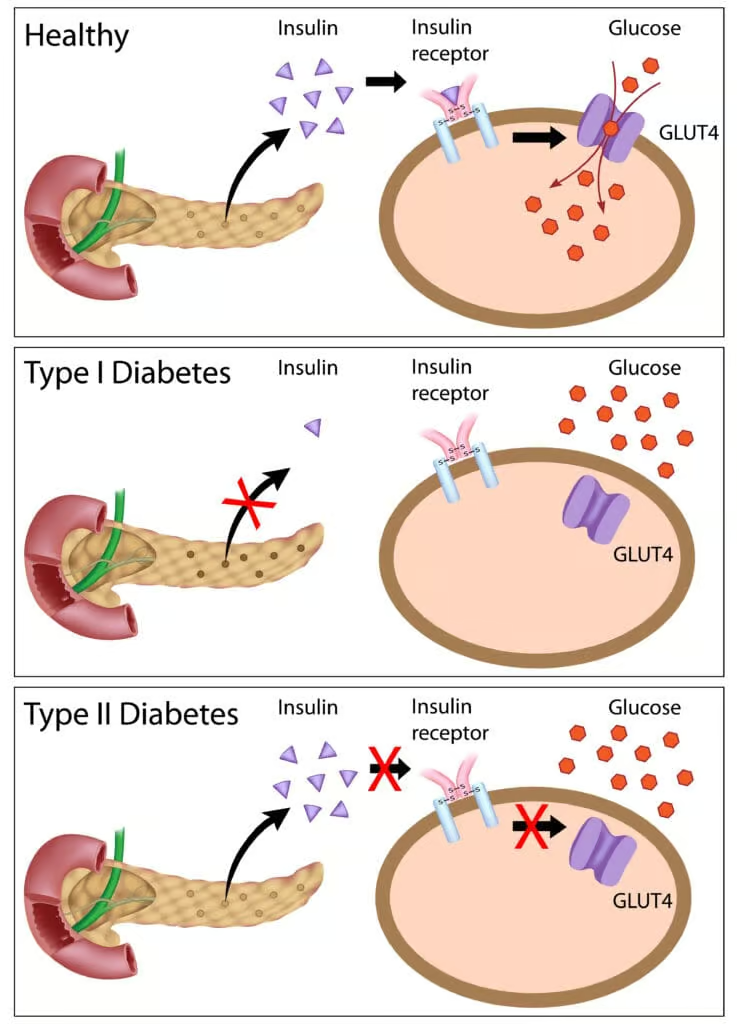
In T2DM, chronic hyperglycemia arises from a complex interplay between genetic predisposition, environmental factors (e.g., obesity, sedentary lifestyle), and metabolic dysregulation. Key pathophysiological hallmarks include:
- Insulin Resistance: Peripheral tissues, especially muscle and adipose tissue, display diminished insulin responsiveness, reducing glucose uptake and utilization.
- Beta-Cell Dysfunction: Pancreatic beta cells fail to compensate by increasing insulin production adequately, partly due to glucotoxicity and lipotoxicity.
- Hepatic Glucose Overproduction: Increased gluconeogenesis and glycogenolysis in the liver exacerbate fasting and postprandial hyperglycemia.
- Dysregulated Incretin System: Decreased levels and/or response to incretins (e.g., GLP-1) hinder insulin secretion and promote excessive glucagon release.
The progressive nature of T2DM calls for a dynamic and often multi-drug approach focused on reversing or compensating for these defects. Lifestyle modifications, including medical nutrition therapy and regular physical activity, form the foundation for therapy, alongside pharmacological agents.
Goals of Pharmacotherapy
The overarching aims of T2DM pharmacotherapy include:
- Reducing Hyperglycemia: Achieving and maintaining a target glycated hemoglobin (HbA1c) level to minimize microvascular complications. Typical targets may be <7.0% for many adults, though this can be individualized.
- Improving Metabolic Parameters: Addressing cardiovascular risk by managing dyslipidemia, hypertension, and body weight.
- Preserving or Improving Beta-Cell Function: Delaying the decline in endogenous insulin secretion, if possible.
- Preventing or Delaying Complications: Lessening the burden of nephropathy, retinopathy, neuropathy, and cardiovascular disease.
- Enhancing Quality of Life: Minimizing side effects, optimizing convenience, and improving patient adherence and satisfaction.
Therapy must be personalized, considering patient factors such as age, comorbidities, duration of diabetes, risk of hypoglycemia, and treatment preferences.
Biguanides (Metformin)
Mechanism of Action
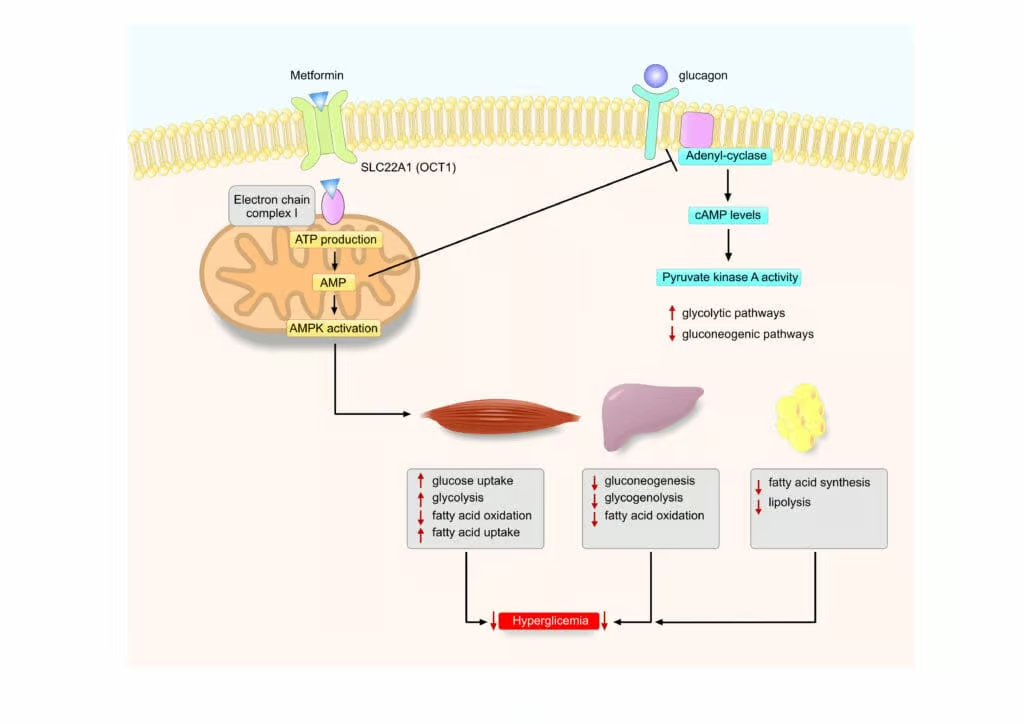
Metformin (a biguanide) is recommended as the first-line oral agent for T2DM management (unless contraindicated). Its primary actions include:
- Decreased Hepatic Gluconeogenesis: Metformin suppresses hepatic glucose production, a key driver of fasting hyperglycemia.
- Enhancement of Insulin Sensitivity: Metformin upregulates peripheral glucose uptake, partially through the activation of AMP-activated protein kinase (AMPK).
- Favorable Weight Profile: By reducing hepatic glucose output and improving insulin sensitivity, metformin generally promotes mild weight loss or weight neutrality.
Efficacy and Clinical Role
Metformin reduces HbA1c levels by approximately 1.0-1.5%. It is also associated with potential cardiovascular benefits, including lower rates of myocardial infarction in overweight/obese T2DM patients. Guidelines widely endorse metformin as initial monotherapy, continuing its use in combination regimens unless significant side effects or contraindications arise.
Adverse Effects and Contraindications
- Gastrointestinal Distress: Diarrhea, abdominal discomfort, and flatulence are relatively common, often mitigated by dose titration and using extended-release formulations.
- Lactic Acidosis: Rare but potentially fatal. Risk increases in renal insufficiency, advanced congestive heart failure, or severe liver disease. Current guidelines suggest caution and dose adjustment if eGFR <30 mL/min/1.73 m².
- Vitamin B12 Deficiency: Long-term use can reduce B12 absorption. Periodic screening is recommended.
Sulfonylureas
Mechanism of Action
Sulfonylureas (e.g., Glibenclamide, Glipizide, Gliclazide, Glimepiride) initiate insulin release by binding to the sulfonylurea receptor 1 (SUR1) on pancreatic beta cells:
- Closure of K-ATP Channels: Accelerates cell depolarization.
- Opening of Voltage-Gated Calcium Channels: Calcium influx stimulates exocytosis of insulin-containing granules.
Clinical Benefits and Limitations
- Efficacy: Sulfonylureas lower HbA1c by about 1-2%. They are cost-effective and widely available.
- Risks: Main adverse effects include hypoglycemia and weight gain. Elderly patients risk severe hypoglycemic episodes, especially with long-acting agents like glibenclamide.
- Treatment Position: Although still frequently used, sulfonylureas rank below metformin and newer agents (like SGLT2 inhibitors or GLP-1 receptor agonists) for long-term therapy. They remain an option for cost-conscious patients or those intolerant to other treatments.
Specific Agents
- Glimepiride: May have a lower incidence of hypoglycemia and once-daily dosing.
- Gliclazide: Often perceived as safer with fewer hypoglycemic episodes, widely used in many countries.
- Glipizide: Shorter half-life, potentially less risky in elderly populations.
Glinides (Meglitinides)
Mechanism of Action
Glinides (e.g., Repaglinide, Nateglinide) work similarly to sulfonylureas by binding to beta-cell SUR receptors but at a slightly different site. They:
- Stimulate Rapid, Short-Lived Insulin Release: Particularly effective at controlling postprandial glucose excursions.
- Short Half-Life: Delivers flexible dosing around meals to target mealtime hyperglycemia.
Clinical Use
- HbA1c Reduction: Around 0.5-1.5%, somewhat less potent than sulfonylureas.
- Advantages: Lower hypoglycemia risk if a meal is skipped or delayed, due to fast onset and short action.
- Disadvantages: Requires multiple daily doses, leading to adherence issues. Also can cause weight gain.
Thiazolidinediones (TZDs)
Mechanism of Action
Thiazolidinediones (e.g., Pioglitazone, Rosiglitazone) activate the nuclear receptor peroxisome proliferator-activated receptor-gamma (PPAR-γ), resulting in:
- Improved Peripheral Insulin Sensitivity: Enhanced adipocyte differentiation and decreased lipotoxicity.
- Reduced Hepatic Gluconeogenesis: Indirectly lowers hepatic glucose output.
- Possible Cardiometabolic Benefits: Pioglitazone may have a favorable effect on lipid profiles, reducing triglycerides.
Efficacy and Concerns
- Glycemic Effect: TZDs lower HbA1c by 0.5-1.4%. They also have durable insulin-sensitizing effects.
- Weight Gain: A major limitation, partly from fluid retention and increased subcutaneous adiposity.
- Edema and Heart Failure Risk: Worsening or new-onset heart failure can occur; caution in patients with reduced ejection fraction.
- Bone Fractures: Increased fracture risk, especially in postmenopausal women.
- Bladder Cancer: Pioglitazone has had controversial links to possible increased risk. Evidence remains mixed.
Alpha-Glucosidase Inhibitors
Mechanism of Action
Agents like Acarbose and Miglitol inhibit intestinal alpha-glucosidases, delaying carbohydrate absorption:
- Postprandial Glucose Reduction: Flattening spikes in blood glucose after meals.
- No Direct Effect on Insulin Secretion: Minimizes risk of hypoglycemia unless combined with other secretagogues or insulin.
Clinical Impact
- Modest HbA1c Reduction: ~ 0.5-1.0%.
- Side Effects: Flatulence, bloating, diarrhea related to undigested carbohydrates fermenting in the colon.
- Optimal Use: Best for patients with mild hyperglycemia focused around meals or those with predominant postprandial elevations. Rarely used as first-line, often used as add-on therapy.
DPP-4 Inhibitors (Gliptins)
Mechanism of Action
Dipeptidyl peptidase-4 (DPP-4) rapidly degrades incretin hormones (GLP-1, GIP) that stimulate insulin release and suppress glucagon. DPP-4 inhibitors (e.g., Sitagliptin, Vildagliptin, Linagliptin, Saxagliptin, Alogliptin) block this enzyme:
- Increased Endogenous GLP-1 and GIP Levels: Enhanced, glucose-dependent insulin secretion and suppressed glucagon.
- Weight Neutrality: Typically do not cause significant weight change.
Clinical Performance
- HbA1c Lowering: ~0.5-1.0%. Effective especially in early T2DM or mild hyperglycemia.
- Tolerability: Generally safe with low risk of hypoglycemia. Rare reports of pancreatitis or joint pains.
- Cardiovascular Effects: Large trials indicate neutral or modest CV benefit. Not as pronounced as SGLT2 inhibitors or GLP-1 receptor agonists.
SGLT2 Inhibitors
Mechanism of Action
Sodium-Glucose Cotransporter 2 (SGLT2) inhibitors (e.g., Empagliflozin, Canagliflozin, Dapagliflozin, Ertugliflozin) block glucose reabsorption in the proximal renal tubule:
- Glycosuria: Promotes urinary excretion of glucose, lowering plasma glucose levels.
- Weight Reduction: Through caloric loss in the urine.
- Blood Pressure Reduction: Osmotic diuresis helps lower blood pressure, valuable in hypertensive T2DM patients.
Clinical Benefits
- Substantial Evidence of Cardiovascular and Renal Protection: Trials (e.g., EMPA-REG OUTCOME, CANVAS) show empagliflozin and canagliflozin significantly reduce cardiovascular events and slow progression of diabetic nephropathy in high-risk patients.
- HbA1c Lowering: ~0.5-1.0%, though potentially higher with baseline hyperglycemia.
- Side Effects: Genitourinary infections (mycotic), possible euglycemic ketoacidosis, volume depletion, and rare diabetic ketoacidosis in T2DM. Canagliflozin has an associated risk of lower limb amputations in some analyses, though data remain debated.
Therapeutic Position
Due to strong CV and renal protection data, SGLT2 inhibitors are now recommended as key second-line or even first-line therapy in T2DM patients with established atherosclerotic cardiovascular disease, heart failure, or chronic kidney disease.
GLP-1 Receptor Agonists
Mechanism of Action
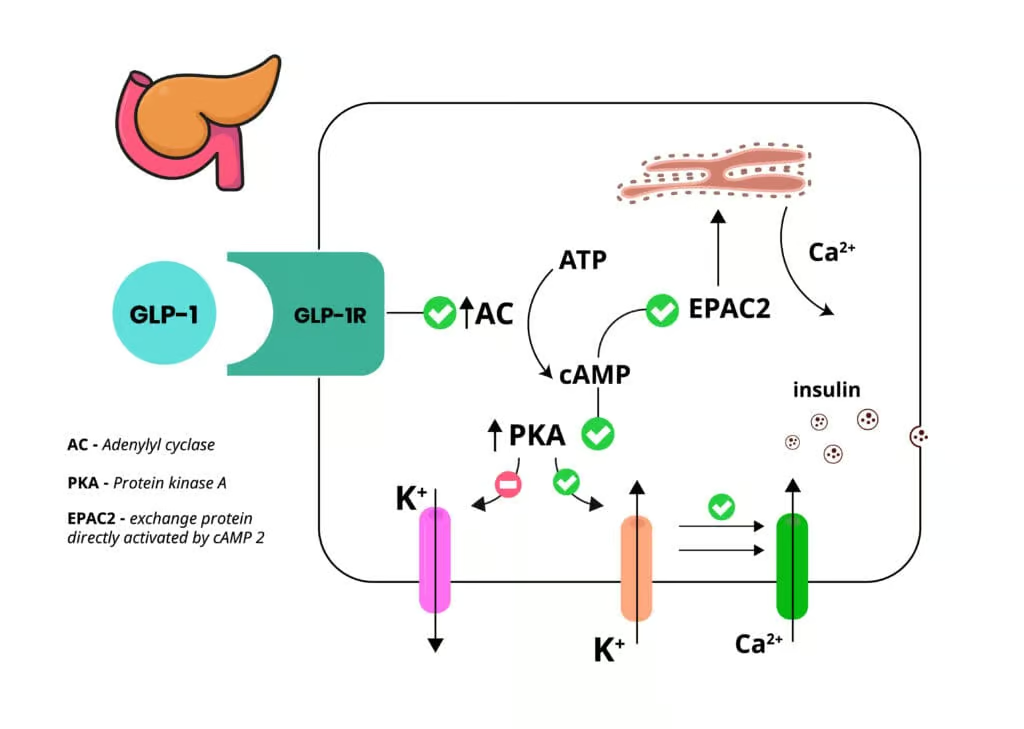
Glucagon-like peptide-1 (GLP-1) receptor agonists (e.g., Exenatide, Liraglutide, Semaglutide, Dulaglutide, Lixisenatide) mimic endogenous GLP-1:
- Glucose-Dependent Insulin Secretion: Boosts insulin release only when plasma glucose is high, minimizing hypoglycemia.
- Glucagon Suppression: Lowers hepatic glucose output.
- Slowed Gastric Emptying: Enhances satiety, leading to weight loss.
- Beta-Cell Preservation: Possible protective effect on beta-cell mass/function.
Clinical Efficacy
- HbA1c Reduction: Typically 1.0-1.5%.
- Weight Loss: A major advantage over most other injectables (like insulin).
- Cardiovascular Outcomes: Trials show liraglutide, semaglutide, and dulaglutide all significantly decrease major adverse cardiovascular events (MACE) in high-risk T2DM populations.
- Side Effects: Gastrointestinal effects (nausea, vomiting) are common, especially early in therapy. Rare but notable risks include pancreatitis and possibly thyroid C-cell tumors in animal studies.
Treatment Role
GLP-1 receptor agonists have emerged as a top choice for T2DM patients who require robust glycemic control, especially if obesity and cardiovascular disease are present. Long-acting, once-weekly preparations (e.g., Semaglutide SC, Dulaglutide) improve adherence and convenience.
Insulin Therapy in Type 2 Diabetes
Indications
Although T2DM is primarily insulin-resistant, exogenous insulin becomes essential in various scenarios:
- Severe Hyperglycemia: Symptomatic or catabolic states (e.g., weight loss, ketonuria) requiring immediate glycemic control.
- Failure of Oral/Injectable Agents: Persistent elevated HbA1c despite maximal combination therapy.
- Acute Illness or Perioperative Period: Short-term insulin coverage during hospitalization or surgeries.
- Pregnancy: Insulin remains the safest glucose-lowering agent with no teratogenic risk.
Types of Insulin
- Basal Insulin: Long-acting forms like Insulin Glargine, Insulin Detemir, Insulin Degludec. Provide 24+ hour steady coverage.
- Prandial (Bolus) Insulin: Rapid-acting analogs (Insulin Lispro, Aspart, Glulisine) or short-acting regular insulin to control postprandial surges.
- premixed Preparations: Combine intermediate-acting with short/rapid insulin in fixed ratios, simplifying regimens but reducing dosing flexibility.
Titration and Monitoring
- Start Low, Go Slow: Typical initial basal insulin addition might begin at 10 units daily or 0.1-0.2 units/kg/day, titrated per fasting glucose.
- Self-Monitoring of Blood Glucose (SMBG): Vital for dose adjustments, preventing hypoglycemia.
- HbA1c: Reevaluated every 3 months to gauge overall control.
Adverse Effects
- Hypoglycemia: Especially with tighter targets or inappropriate dosing.
- Weight Gain: Insulin is anabolic, promoting adipose deposition.
- Injection Burden: Physical and psychological demands of insulin injections or infusion pumps.
Insulin remains the most potent glucose-lowering agent, with the flexibility to manage even advanced T2DM, though at the cost of possible hypoglycemia and weight gain.
Combination Therapy and Stepwise Approaches
Standard Progression
- Lifestyle + Metformin: Foundation of T2DM therapy.
- Add Second-Line Drug: SGLT2 inhibitor or GLP-1 receptor agonist if high CV or renal risk; otherwise DPP-4 inhibitor, TZD, or sulfonylurea.
- Triple Therapy: If targets are unmet, add a third agent (e.g. combining SGLT2 inhibitor + GLP-1 receptor agonist or adding basal insulin).
- Intensification: Progress to multiple daily injections of insulin, especially in advanced beta-cell failure.
Tailored Regimens
Patient-specific factors (comorbidity, cost, preference, route of administration) guide the choice of second or third agent. Recently, guidelines emphasize early introduction of SGLT2 inhibitors or GLP-1 receptor agonists in patients with established atherosclerotic heart disease, heart failure, or kidney disease, regardless of baseline HbA1c. Combining these classes can maximize glycemic and cardioprotective benefits but must be balanced against cost considerations and potential side effects.
Special Populations
Older Adults
- Hypoglycemia Risk: Caution with sulfonylureas, insulin, or aggressive targets.
- Renal Function: Dose adjustments or avoidance of metformin if eGFR <30 mL/min/1.73 m², watch for SGLT2 inhibitors if eGFR is severely reduced.
- Polypharmacy: Simplify regimens to improve adherence, minimize potential drug interactions.
Pregnancy
- Insulin remains the mainstay for T2DM or gestational diabetes. Some guidelines permit use of metformin or glyburide but with caution.
- GLP-1 RAs, SGLT2 inhibitors, and other newer agents lack sufficient safety data in pregnancy.
Heart Failure
- Certain T2DM drugs (TZDs) can exacerbate heart failure.
- SGLT2 inhibitors hold strong evidence for improving HF outcomes.
- Evaluate volume status and monitor for euglycemic ketoacidosis in advanced HF patients on SGLT2 inhibitors.
Chronic Kidney Disease (CKD)
- SGLT2 inhibitors show renal protection, recommended if eGFR is above threshold (most recommended above 30 mL/min/1.73 m²).
- Dose Adjustments: Many agents (metformin, DPP-4 inhibitors, insulin) require modifications as eGFR declines.
Patient Education and Lifestyle Support
Adherence and Self-Monitoring
Pharmacotherapy success hinges on informed, empowered patients who understand:
- Medication Timing: e.g., glinides before meals, once-weekly GLP-1 injections, etc.
- Hypoglycemia Prevention: Recognizing signs, carrying glucose tablets, adjusting doses for exercise.
- Dietary Interventions: Coordinating carbohydrate intake with insulin or secretagogues.
Weight Management
- Agents like GLP-1 receptor agonists and SGLT2 inhibitors facilitate weight loss or neutral impact.
- Emphasize calorie balance, mindful eating, and structured physical activity to optimize metabolic control.
Self-Care and Comorbidity Management
- Blood Pressure Control: Use of ACE inhibitors or ARBs, especially for nephroprotection.
- Lipids: Statins recommended for most T2DM patients.
- Smoking Cessation: Minimizes cardiovascular and microvascular risks.
Emerging Therapies and Future Directions
Novel Agents
Research focuses on:
- Dual or Triple Incretin Receptor Agonists: e.g., GLP-1/GIP co-agonists for enhanced weight reduction and glycemic control.
- Bile Acid Sequestrants, Dopamine Agonists: Present but with modest efficacy.
- Cellular and Gene Therapies: Beta-cell transplantation or gene editing remain exploratory.
Personalized Medicine
Genetic profiling, biomarkers, and advanced glucose monitoring technologies help tailor regimens. Real-time continuous glucose monitoring (CGM) fosters dynamic insulin dose adjustments or immediate feedback for lifestyle changes.
Artificial Pancreas Systems
Integrating CGM with closed-loop insulin delivery advances T1DM care, with potential utility for some T2DM populations on intensive insulin therapy.
Real-World Data and Cost Considerations
Healthcare systems weigh the high cost of SGLT2 inhibitors, GLP-1 analogs, or insulin analogs against robust improvements in outcomes. Comparative effectiveness research influences coverage decisions and patient access.
Conclusion
Type 2 Diabetes Mellitus pharmacotherapy has evolved from a narrow group of oral agents (sulfonylureas, metformin) to an expansive selection of drug classes, each addressing distinct pathophysiological targets. Metformin remains the cornerstone of first-line therapy for most, complemented by SGLT2 inhibitors or GLP-1 receptor agonists in patients where cardiovascular or renal protection is paramount. Over time, the appropriate combination or escalation to insulin becomes critical when glycemic targets remain elusive or beta-cell function deteriorates.
The entire therapeutic plan is anchored in patient-centered care, integrating medication efficacy, safety, cost, and comorbidities. Medications such as sulfonylureas, DPP-4 inhibitors, and TZDs still play roles, though concerns about hypoglycemia, weight gain, or edema can limit their usage relative to newer agents. In parallel, lifestyle interventions including nutritional support, physical activity regimens, and behavioral modifications are indispensable at every stage.
Looking ahead, the surge of innovative agents (e.g., dual incretin agonists) and advanced technologies (like closed-loop insulin delivery) offers hope for even more personalized, safe, and effective T2DM care. The guiding principles of addressing insulin resistance, preserving beta-cell function, and preventing complications through a multi-pronged approach remain paramount, underscoring the complexity and importance of ongoing research and clinical vigilance in T2DM management.
Book Citations
- Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 13th Edition.
- Katzung BG, Basic & Clinical Pharmacology, 15th Edition.
- Rang HP, Dale MM, Rang & Dale’s Pharmacology, 8th Edition.