Introduction
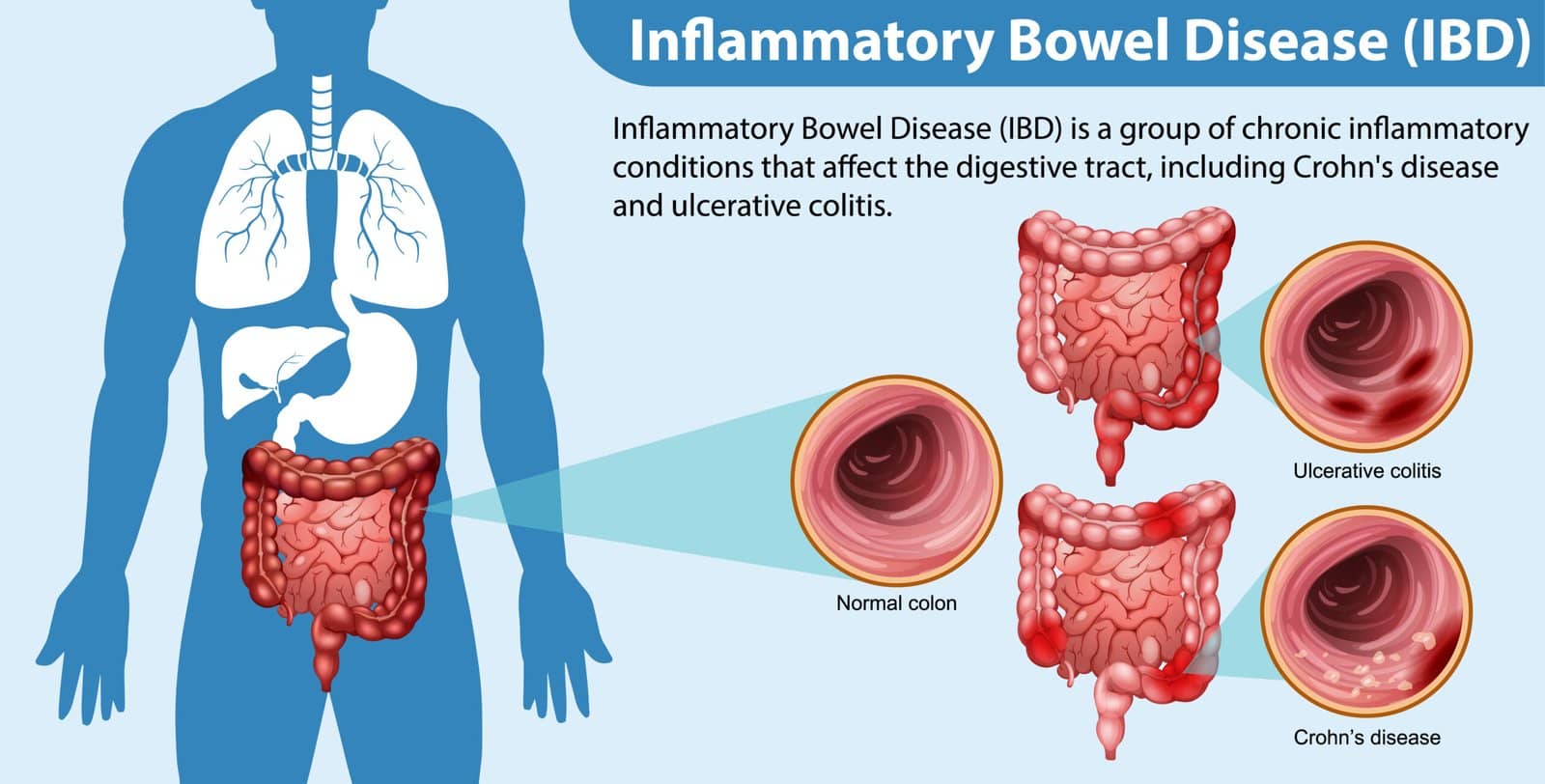
Inflammatory Bowel Disease (IBD) is a chronic, relapsing-remitting inflammatory disorder of the gastrointestinal tract that primarily includes Crohn’s disease (CD) and ulcerative colitis (UC). Both conditions can produce significant morbidity, including abdominal pain, diarrhea (often with blood), weight loss, and systemic manifestations such as anemia. Thought to arise via an intricate interplay of genetic predisposition, immune dysregulation, microbial factors, and environmental triggers, IBD requires long-term management strategies that address symptom control and prevention of complications. This article provides an overview of the approaches to managing IBD, with an emphasis on evidence-based treatments. While both UC and CD have distinct pathophysiological and clinical features, they share overlapping treatment modalities that focus on modulating the dysregulated immune response in the gut. By understanding the range of available therapies—from conventional glucocorticoids to emerging biologic agents—clinicians can individualize patient care.
Treatment selection depends on multiple factors, including disease location, severity, complications, and comorbidities. In mild cases, optimized nutrition and simple anti-inflammatory medications may be enough. Moderately active or severe disease often necessitates more potent therapies such as immunomodulators or biologics. In certain contexts—especially in the presence of complicating strictures, fistulas, or non-healing disease—surgical consultation plays a pivotal role. The ultimate aim of IBD management strategies is not only clinical remission but also mucosal healing, thereby preventing long-term complications, improving quality of life, and reducing the need for hospitalizations and surgeries.
Pathophysiological Rationale for Therapeutic Approaches
Central to the pathogenesis of IBD is an exaggerated immune response against intestinal flora within a genetically susceptible host. Immune cells, including T lymphocytes, B lymphocytes, and various innate immune effectors, drive persistent inflammation in the intestinal mucosa. Pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-12, IL-23, and others are highly implicated in this immune cascade. This enriched cytokine milieu results in tissue damage, ulcers, and, eventually, fibrosis if inflammation persists over the long term.
In UC, inflammation is confined to the colonic mucosa and submucosa, typically involving only the superficial layers. In contrast, CD can affect the entire gastrointestinal tract from mouth to anus, often presenting with transmural inflammation that can result in complications such as strictures, fistulas, and deep ulcers. Modern treatments address both the underlying immune dysregulation and the disease’s downstream complications. For instance, aminosalicylates curb local inflammation in mild to moderate UC, while potent immunomodulators and biologics aim to block specific cytokines or cells that drive the inflammatory process. Therapeutic decisions are best guided by a thorough clinical evaluation, endoscopic findings, and biomarkers like fecal calprotectin, which can monitor inflammatory burden and predict relapses.
General Principles of Medical Management
Medical management of IBD is stepwise and is informed by disease severity, localization, and extraintestinal manifestations. Broadly, this management can be segmented into several categories:
- Induction of Remission – Achieving symptomatic relief and minimizing inflammation.
- Maintenance of Remission – Preventing relapse through ongoing therapy, dose adjustments, or step-down strategies when appropriate.
- Management of Complications – Addressing strictures, abscesses, fistulas, malnutrition, and extraintestinal issues like arthritis or primary sclerosing cholangitis.
An important component of therapy is ongoing patient education. Patients must be instructed on medication adherence, dietary management, and vigilant recognition of exacerbating factors (e.g., smoking in CD). Collaborative, multidisciplinary care—often involving gastroenterologists, primary care physicians, surgeons, dietitians, and mental health professionals—provides the best outcomes.
Aminosalicylates and Corticosteroids
Aminosalicylates (5-aminosalicylic acid, or 5-ASA) are often first-line therapies in mild to moderate ulcerative colitis. These drugs reduce local inflammation within the colonic lumen by inhibiting cyclooxygenase and lipoxygenase pathways to diminish prostaglandin and leukotriene production. Oral and topical formulations are commonly used. Though they play a more prominent role in UC, 5-ASA may occasionally be considered for mild Crohn’s disease with colonic involvement, although data supporting their efficacy in CD are less robust.
For patients with moderate to severe flares, or those who do not respond adequately to 5-ASA, corticosteroids (e.g., prednisone, budesonide) are a mainstay for inducing remission. Steroids provide more potent anti-inflammatory action by broadly suppressing cytokine production. Oral prednisone is commonly used in outpatient settings, while intravenous hydrocortisone or methylprednisolone may be required for acute severe exacerbations needing hospitalization. Budesonide, a corticosteroid with high first-pass metabolism, offers favorable safety profiles in mild to moderate disease, especially involving the ileum and right colon. However, steroids are not ideal for long-term maintenance due to adverse effects such as weight gain, hyperglycemia, osteoporosis, and risk of infections.
Immunomodulators
Immunomodulators—such as azathioprine, 6-mercaptopurine (6-MP), and methotrexate—offer steroid-sparing properties and are frequently used in patients who require repeated steroid courses or have moderate to severe disease. These agents target specific pathways in immune cell proliferation, thereby limiting pro-inflammatory activity over time. For example:
- Azathioprine (AZA) and 6-MP: Purine analogs that interfere with cellular DNA and RNA synthesis, leading to a reduction in T-cell and B-cell proliferation. They are effective in maintaining remission in both UC and CD but require monitoring for hepatotoxicity, bone marrow suppression, and risk of malignancy in long-term use.
- Methotrexate: An antimetabolite that inhibits folate-dependent enzymatic reactions. It is often used off-label for CD, especially in patients who cannot tolerate or do not respond to AZA/6-MP. Methotrexate requires routine monitoring of hepatic function and blood counts as well.
These immunomodulators can take several weeks or months before clinically significant remission is achieved. They are particularly useful in combination with biologics, enhancing effectiveness and reducing the risk of anti-drug antibody formation.
Biologic Therapies
Biologic agents have revolutionized the management of moderate to severe IBD. These agents are antibodies or fusion proteins designed to block specific pro-inflammatory mediators. Over the past two decades, the development and use of biologics have dramatically improved outcomes, reducing hospitalization rates and surgical needs. Four main classes of biologic therapy are commonly employed in IBD:
- Anti-TNF Agents (e.g., infliximab, adalimumab, certolizumab pegol): These drugs inhibit tumor necrosis factor (TNF)-α, a central cytokine in IBD pathogenesis. They are effective in inducing and maintaining remission, closing fistulas (particularly in Crohn’s), and promoting mucosal healing. However, they carry an increased risk for infections (particularly reactivation of latent tuberculosis) and certain malignancies.
- Anti-Integrin Agents (e.g., vedolizumab): These selectively block leukocyte migration into the inflamed intestine, thereby reducing localized GI inflammation.
- Anti-IL-12/23 Agents (e.g., ustekinumab): By targeting the p40 subunit shared by IL-12 and IL-23, these agents modulate both innate and adaptive immune pathways. They have demonstrated efficacy in moderate to severe Crohn’s and ulcerative colitis.
- JAK Inhibitors and Other Small Molecules (e.g., tofacitinib): While technically not “biologic monoclonal antibodies,” these oral small molecules inhibit Janus Kinase (JAK) pathways, crucial for cytokine signaling. They are increasingly used in moderate to severe ulcerative colitis patients.
Selecting a biologic is a thoughtful process involving disease phenotype, severity, previous medication history, presence of extraintestinal manifestations, and patient-specific factors such as comorbidities or preferences regarding administration (e.g., intravenous vs. subcutaneous).
Nutritional Management
Nutrition plays a vital and sometimes overlooked role in IBD management. Patients with increased inflammatory burden and malabsorption risk may require close monitoring of micronutrient levels such as iron, vitamin B12, folate, and vitamin D. Dietary modifications may help alleviate some gastrointestinal symptoms, although there is no single “IBD diet” proven to induce remission consistently.
In children and adolescents with Crohn’s disease, exclusive enteral nutrition (EEN) has been utilized as a strategy to induce remission while preserving growth and reducing steroid exposure. While its application in adults is limited by adherence challenges, EEN underscores the importance of nutritional therapy. For both pediatric and adult patients, supplementation with specific nutrients can be crucial. Calcium and vitamin D supplementation are often indicated to counteract the effects of corticosteroids on bone density. Periodic screening for malnutrition ensures early intervention, which can significantly improve clinical outcomes and patient quality of life.
Surgical Interventions
Although medical therapy remains the cornerstone of IBD management, approximately 20-30% of patients with UC and up to 70-80% of patients with Crohn’s disease eventually require surgical intervention at some point in their lives. The indications for surgery may include:
- Refractory Disease: When medical therapy is unable to control symptoms or achieve mucosal healing.
- Complications: Including strictures, fistulas, perforations, or massive hemorrhage.
- Dysplasia or Cancer: Patients with chronic colitis have an increased risk of colorectal cancer, and confirmed high-grade dysplasia or invasive cancer necessitates surgical resection.
Surgical procedures vary by IBD subtype. In UC, a proctocolectomy with ileal pouch-anal anastomosis (IPAA) can be curative by removing all diseased colonic mucosa. However, complications like pouchitis can occur. In CD, surgical approaches aim to address complications (strictureplasty, drainage of abscesses, segmental resections) while preserving as much bowel as possible to avoid short-bowel syndrome. Minimally invasive techniques are increasingly employed to decrease postoperative complications and hasten recovery.
Emerging Therapies and Future Directions
The therapeutic landscape of IBD continues to evolve. Ongoing research targets more precise and individualized treatments by identifying biomarkers predictive of response. Novel small molecules enriching the JAK-STAT pathway inhibition, S1P receptor modulators (e.g., ozanimod), and expanding classes of biologics targeting novel pathways (e.g., IL-17, IL-10, B-cell or T-cell surface markers) show promise in ongoing clinical trials.
Personalized medicine, guided by genetic profiling and molecular signatures, may one day tailor therapy to the phenotype and genotype of individual patients. Additionally, microbiome research is revealing how manipulations of intestinal flora—whether through dietary interventions, probiotics, or fecal microbiota transplant—could be applied to bolster remission. While the role of fecal microbiota transplantation in UC is more established than in Crohn’s, it remains an area of intense investigation.
Non-Pharmacological and Supportive Care
Psychosocial support is pivotal for many patients, as the unpredictable nature of IBD flare-ups can result in significant anxiety and depression. Stress management, mental health counseling, and community support groups can all help improve adherence and coping mechanisms. Regular exercise within a person’s tolerance level may also contribute to improved well-being and reduced mucosal inflammation, although evidence is still emerging.
For individuals with extraintestinal manifestations (e.g., joint pain, skin lesions, primary sclerosing cholangitis), co-management with rheumatologists, dermatologists, and hepatologists can help optimize overall health. Smoking cessation is particularly important for Crohn’s disease patients, as smoking exacerbates disease activity and recurrence. Finally, regular colonoscopic surveillance is recommended to detect dysplasia in individuals with long-standing colonic involvement.
Conclusion
Management of Inflammatory Bowel Disease is multifaceted and requires a personalized, comprehensive approach. From 5-ASA medications to advanced biologic agents, therapeutic strategies must be tailored to each patient’s disease severity, distribution, and treatment response. Monitoring disease activity through clinical, endoscopic, and biochemical markers is essential for treatment adjustments. In many instances, combination therapy allows for both efficient induction of remission and long-term maintenance. Meanwhile, surgery retains an important role for those who fail medical therapy or who develop complications.
A holistic approach—encompassing dietary optimization, psychosocial support, and surveillance for complications—will position patients for the best possible outcomes. Going forward, research into precision medicine, novel pharmacologic targets, and microbiome manipulation may transform the management of IBD to become even more targeted and individualized. With these evolving modalities, the ultimate goals remain the same: to reduce symptom burden, maintain remission, prevent complications, and preserve health-related quality of life in this chronic, often life-altering condition.
References
- Kirsner JB, Feuerstein JD, editors. Kirsner’s Inflammatory Bowel Diseases. 7th ed. Philadelphia: Saunders; 2019.
- Podolsky DK, Camilleri M, Fitz JG, Kalloo AN, Shanahan F, Wang TC, editors. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. 10th ed. Philadelphia: Elsevier Saunders; 2016.
- Goldman L, Schafer AI, editors. Goldman-Cecil Medicine. 25th ed. Philadelphia: Elsevier Saunders; 2016.