Introduction
Vancomycin is a prominent glycopeptide antibiotic widely recognized for its efficacy in treating severe infections caused by Gram-positive bacteria, particularly methicillin-resistant Staphylococcus aureus (MRSA) and certain Clostridioides difficile (C. diff) infections. Its critical role in modern medicine stems from an increasing need to address challenging and resistant microbial pathogens that threaten patient safety in hospitals and community settings. Discovered in the 1950s, vancomycin’s clinical use evolved over time, largely reserved for severe infections not amenable to treatment with other agents. Today, its utility extends beyond the management of MRSA, covering a range of resistant Gram-positive organisms.
In this article, we dive deeply into the pharmacology of vancomycin, touching on its history, mechanism of action, pharmacokinetics, therapeutic monitoring, dosing considerations, side effects, resistance mechanisms, ongoing research, and best practices. By the end, you will have a solid understanding of how vancomycin functions, why it is pivotal in managing resistant infections, and how clinicians optimize its use to minimize toxicity and prevent the emergence of further resistance.
Historical Development and Discovery
The journey of vancomycin began in the 1950s when it was first isolated from a soil sample collected in the jungles of Borneo. Scientists at Eli Lilly were searching for novel antimicrobials in response to increasing resistance patterns. They identified a compound produced by the bacterium Amycolatopsis orientalis (previously known as Streptomyces orientalis) that demonstrated potent activity against Gram-positive bacteria.
Early manufacturing runs of vancomycin resulted in an impure product, initially earning it the nickname “Mississippi mud” due to its brownish appearance. Refinements in production techniques eventually yielded a purer formulation, which helped reduce the incidence of contaminant-related side effects. Over time, as the prevalence of methicillin-resistant organisms rose, vancomycin emerged as the gold-standard therapy for serious infections, such as MRSA pneumonia, complicated skin and soft tissue infections, and endocarditis caused by resistant staphylococcal strains.
Despite the introduction of newer antimicrobial agents, vancomycin remains one of the most important antibiotics for clinicians dealing with hospital-acquired infections. Its continued relevance underscores the importance of understanding its pharmacological characteristics, from mechanism of action to administration strategies.
Classification and Chemical Structure of Vancomycin
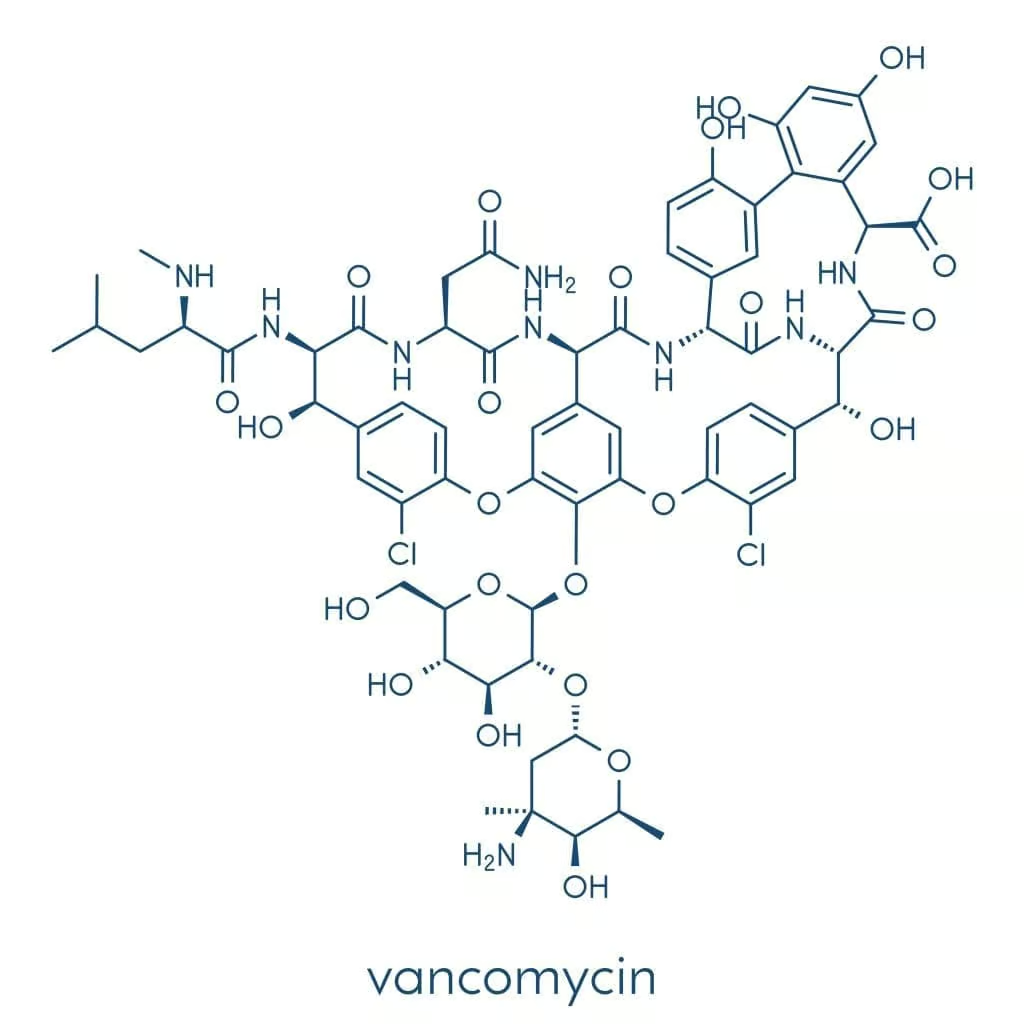
Vancomycin is classified as a glycopeptide antibiotic, characterized by its large molecular weight and specialised chemical structure composed of a heptapeptide core linked to sugar moieties. This structure is distinct from beta-lactam antibiotics like penicillins and cephalosporins. Instead of targeting the penicillin-binding proteins (PBPs), vancomycin operates at a different juncture in bacterial cell wall synthesis, as discussed in more detail below.
Glycopeptide antibiotics, including vancomycin and its newer derivatives (e.g., teicoplanin, telavancin, dalbavancin, and oritavancin), share a central ring or peptide-based scaffold attached to various side chains. These chemical arrangements dictate how effectively each agent binds to bacterial cell wall precursor units and resists enzymatic degradation, as well as how the drug is metabolized and excreted by the body.
Chemically, vancomycin is a complex molecule, poorly absorbed from the gastrointestinal tract. Consequently, for systemic infections, it is given almost exclusively intravenously to ensure therapeutic levels in the bloodstream and tissues. However, for the management of intestinal infections such as C. difficile colitis, oral vancomycin plays a central role, acting locally in the digestive tract against this challenging pathogen.
Vancomycin’s mechanism of action lies at the heart of its remarkable efficacy against resistant Gram-positive microbes. Unlike beta-lactam antibiotics that disrupt cell wall synthesis by binding to PBPs, vancomycin interferes at an earlier stage. Specifically, it targets the D-alanyl-D-alanine (D-Ala-D-Ala) terminal residues on the peptidoglycan precursors that form the bacterial cell wall.
- Binding to D-Ala-D-Ala: By binding to the D-Ala-D-Ala moieties, vancomycin effectively blocks the action of transglycosylase enzymes that cross-link the peptidoglycan layers. This prevents the extension of the peptidoglycan network, compromising cell wall integrity.
- Inhibition of Cross-Linking: Because the cell wall can no longer be sufficiently cross-linked, bacteria become vulnerable to osmotic pressure and eventually lyse.
- High Specificity: Thanks to its detailed three-dimensional conformation, vancomycin demonstrates high specificity for Gram-positive organisms that rely heavily on thick peptidoglycan layers. Gram-negative bacteria, with their thinner peptidoglycan and outer membrane, are inherently more resistant to vancomycin penetration.
Due to this critical interference in bacterial cell wall ecology, vancomycin is often described as a “last line of defense” for certain resistant Gram-positive infections. It is especially valued in treating staphylococcal and streptococcal infections, where its unique binding mechanism helps circumvent the resistance that undermines other antibiotic classes.
Mechanism of Action of Vancomycin
Vancomycin’s mechanism of action lies at the heart of its remarkable efficacy against resistant Gram-positive microbes. Unlike beta-lactam antibiotics that disrupt cell wall synthesis by binding to PBPs, vancomycin interferes at an earlier stage. Specifically, it targets the D-alanyl-D-alanine (D-Ala-D-Ala) terminal residues on the peptidoglycan precursors that form the bacterial cell wall.
- Binding to D-Ala-D-Ala: By binding to the D-Ala-D-Ala moieties, vancomycin effectively blocks the action of transglycosylase enzymes that cross-link the peptidoglycan layers. This prevents the extension of the peptidoglycan network, compromising cell wall integrity.
- Inhibition of Cross-Linking: Because the cell wall can no longer be sufficiently cross-linked, bacteria become vulnerable to osmotic pressure and eventually lyse.
- High Specificity: Thanks to its detailed three-dimensional conformation, vancomycin demonstrates high specificity for Gram-positive organisms that rely heavily on thick peptidoglycan layers. Gram-negative bacteria, with their thinner peptidoglycan and outer membrane, are inherently more resistant to vancomycin penetration.
Due to this critical interference in bacterial cell wall ecology, vancomycin is often described as a “last line of defense” for certain resistant Gram-positive infections. It is especially valued in treating staphylococcal and streptococcal infections, where its unique binding mechanism helps circumvent the resistance that undermines other antibiotic classes.
Pharmacodynamics
Pharmacodynamics refers to the relationship between drug concentration and physiological effect. Vancomycin exhibits time-dependent bactericidal activity, meaning its efficacy correlates with the duration during which the plasma (or tissue) concentration remains above the minimum inhibitory concentration (MIC) for the target bacteria. However, there is also evidence suggesting partial concentration-dependent activity, making it important to consider both the peak levels and time above MIC.Key points in vancomycin pharmacodynamics:
- Bactericidal Effect: Vancomycin typically achieves a bactericidal (killing) effect, particularly against staphylococci and streptococci, though certain enterococci demonstrate variable susceptibility.
- Area Under the Curve (AUC)/MIC Ratio: Numerous clinical guidelines emphasize maintaining an AUC/MIC ratio of at least 400 for MRSA infections. This parameter suggests that both concentration and time above MIC are crucial for successful bacterial eradication.
- Effect on Bacterial Tolerance: In some settings, extended infusion times or continuous infusions are used to sustain adequate serum concentrations, optimizing bactericidal effects while aiming to limit nephrotoxicity.
- Post-Antibiotic Effect (PAE): Vancomycin demonstrates a modest PAE against certain strains of Staphylococcus aureus, indicating that bacterial growth remains suppressed even after drug levels drop below the MIC.
Understanding vancomycin’s pharmacodynamic profile is vital for designing precise dosing regimens that balance therapeutic efficacy against possible toxicities. By ensuring exposures remain within target parameters, clinicians can maximize the antibiotic’s benefits while mitigating harm to patients.
Pharmacokinetics
- Absorption: Oral bioavailability of vancomycin is negligible in healthy individuals. Hence, intravenous administration is imperative when targeting systemic infections. However, in the setting of colitis (particularly C. difficile infection), oral vancomycin exerts local action in the gut without significant systemic absorption.
- Distribution: Once given IV, vancomycin has a volume of distribution ranging from 0.4 to 1.0 L/kg, depending on the patient’s physiology and presence of edema or other factors (e.g., critical illness). It moderately binds to plasma proteins and can penetrate inflamed meninges. Thus, it can be used for CNS infections like staphylococcal meningitis, though achieving target CSF concentrations may require higher dosing or intraventricular administration.
- Metabolism: Exchange of the drug mainly happens in tissues and bodily fluids. Vancomycin does not undergo significant hepatic metabolism, which explains its unchanged excretion profile.
- Elimination: Approximately 80-90% of an administered dose is excreted unchanged via the kidneys, establishing renal clearance as the primary elimination route. As such, vancomycin dosing must be carefully adjusted in patients with kidney dysfunction to prevent accumulation and toxicity.
Given renal excretion is central to vancomycin clearance, monitoring renal function and drug levels (peak and trough or AUC-based levels) is crucial for optimizing therapy and avoiding nephrotoxicity, a well-known hazard of vancomycin misuse.
Clinical Uses of Vancomycin
Its strong antibacterial effect against Gram-positive organisms underpins its widespread clinical use:
- Methicillin-Resistant Staphylococcus aureus (MRSA): Leading the list is MRSA therapy, where vancomycin serves as a frontline agent in severe skin and soft tissue infections, pneumonia, osteomyelitis, and endocarditis. When patients exhibit allergies or resistance to other drugs, vancomycin becomes indispensable for broader Gram-positive coverage.
- Staphylococcal Endocarditis: Vancomycin is recommended for penicillin-allergic patients or for organisms resistant to methicillin. In synergy with aminoglycosides or rifampin, vancomycin can optimize the killing of adherent bacteria on heart valves.
- Severe Streptococcal and Enterococcal Infections: Some Streptococcus species and certain strains of Enterococci that are resistant to beta-lactams or penicillins can respond to vancomycin. However, emerging vancomycin-resistant enterococci (VRE) remain a major concern in hospital settings.
- C. difficile-Associated Diarrhea: While metronidazole used to be first-line for mild to moderate cases, current guidelines often favor oral vancomycin for severe or complicated infections due to superior efficacy and lower failure rates. In these cases, vancomycin’s poor oral absorption becomes an advantage for localized gut action.
Beyond these standard uses, clinicians may resort to vancomycin for other difficult Gram-positive infections. In each scenario, rational prescribing and vigilant monitoring help uphold its efficacy and minimize resistance.
Administration and Dosing Strategies
Optimizing vancomycin dosing can be challenging, as multiple factors—body weight, renal function, site of infection, organism susceptibility—must be considered. Key strategies include:
- Weight-Based Dosing: For adults with normal renal function, an initial IV loading dose of 25-30 mg/kg (based on total body weight) may be employed in critically ill or severe infections to rapidly achieve therapeutic concentrations. Maintenance doses typically range from 15-20 mg/kg every 8-12 hours.
- Renal Adjustment: As kidney function declines, vancomycin clearance decreases. Clinicians adjust dosing intervals or reduce dosage accordingly in patients with acute or chronic kidney disease.
- Therapeutic Drug Monitoring (TDM): Traditional approaches emphasize vancomycin trough levels (e.g., 10-20 μg/mL, depending on infection severity), whereas modern guidelines increasingly recommend AUC-guided dosing (AUC/MIC of at least 400). This approach strives to ensure adequate bacterial kill while reducing nephrotoxic risks.
- Continuous Infusion vs. Intermittent Infusion: Some clinicians opt for continuous infusion to maintain stable serum levels, potentially reducing the risk of nephrotoxicity. However, intermittent infusion remains a standard approach in many settings.
- Oral Vancomycin: For pseudomembranous colitis caused by C. difficile, oral dosing patterns vary from 125-500 mg every 6 hours for about 10-14 days, depending on disease severity.
The complexity of dosing underscores why vancomycin therapy often involves interdisciplinary collaboration among infectious disease specialists, pharmacists, and nurses to refine regimens and responsibly manage the antibiotic’s use.
Therapeutic Drug Monitoring
Given vancomycin’s association with nephrotoxicity and ototoxicity, therapeutic drug monitoring (TDM) is integral to patient management:
• Trough-Level Monitoring: Historically, clinicians measured serum trough levels (taken 30 minutes before the next dose) to ensure they remained above 10 μg/mL for less severe infections or between 15-20 μg/mL for more complicated or deep-seated infections.
• AUC-Based Monitoring: In recent years, focus has shifted to the 24-hour area under the concentration-time curve (AUC). Achieving an AUC/MIC ratio ≥400 is associated with effective bacterial killing. This approach might reduce the emphasis on high troughs that can expose patients to nephrotoxic risks.
• Population Pharmacokinetics: Many hospitals are adopting Bayesian software to estimate individual pharmacokinetic parameters. This reduces the need for frequent blood draws and enhances dose individualization by providing real-time AUC estimates.
• Timing of Monitoring: Levels are often checked early in the course of therapy—after 3-5 doses or sooner if the patient is unstable—and then at regular intervals once steady-state or stable concentrations are reached.
Therapeutic drug monitoring not only helps optimize the clinical outcome for the patient but also indirectly curtails resistant mutant subpopulations by maintaining effective drug levels in tissues.
Side Effects and Toxicities of Vancomycin
Like any potent antibiotic, carries a risk of adverse reactions. Awareness of common and severe toxicities helps clinicians deploy mitigation strategies early:
- Nephrotoxicity: One of the most significant concerns is kidney injury, raising serum creatinine and reducing glomerular filtration rates. Monitoring trough or AUC levels, ensuring adequate hydration, and limiting use of concomitant nephrotoxic agents (e.g., aminoglycosides, NSAIDs) are essential preventive steps.
- Ototoxicity: High vancomycin concentrations may damage cranial nerve VIII, leading to hearing loss or vestibular dysfunction. While less common with modern dosing approaches, the risk persists in patients with existing hearing deficits or when co-administered with other ototoxic drugs.
- Red Man Syndrome: Characterized by flushing, rash, pruritus, and sometimes hypotension, this infusion-related reaction is caused by histamine release. Slowing the IV infusion rate and premedicating with antihistamines usually mitigates this effect.
- Hypersensitivity Reactions: Rare severe allergic reactions such as anaphylaxis, though unusual, can occur.
- Thrombophlebitis: Vancomycin can irritate the veins, potentially leading to redness, swelling, and pain at the infusion site. Using a large peripheral vein or a central venous catheter can help reduce this complication.
By balancing the therapeutic benefits against possible side effects, clinicians can harness vancomycin’s activity safely. Early recognition of symptoms, routine TDM, and supportive measures (like adjusting infusion speed) minimize the risk of adverse outcomes.
Therapeutic Drug Monitoring
Given vancomycin’s association with nephrotoxicity and ototoxicity, therapeutic drug monitoring (TDM) is integral to patient management:
• Trough-Level Monitoring: Historically, clinicians measured serum trough levels (taken 30 minutes before the next dose) to ensure they remained above 10 μg/mL for less severe infections or between 15-20 μg/mL for more complicated or deep-seated infections.
• AUC-Based Monitoring: In recent years, focus has shifted to the 24-hour area under the concentration-time curve (AUC). Achieving an AUC/MIC ratio ≥400 is associated with effective bacterial killing. This approach might reduce the emphasis on high troughs that can expose patients to nephrotoxic risks.
• Population Pharmacokinetics: Many hospitals are adopting Bayesian software to estimate individual pharmacokinetic parameters. This reduces the need for frequent blood draws and enhances dose individualization by providing real-time AUC estimates.
• Timing of Monitoring: Levels are often checked early in the course of therapy—after 3-5 doses or sooner if the patient is unstable—and then at regular intervals once steady-state or stable concentrations are reached.
Therapeutic drug monitoring not only helps optimize the clinical outcome for the patient but also indirectly curtails resistant mutant subpopulations by maintaining effective drug levels in tissues.
Resistance and Vancomycin-Resistant Organisms
The emergence of vancomycin-resistant enterococci (VRE) and, less commonly, vancomycin-resistant Staphylococcus aureus (VRSA), underscores a difficult challenge in infection control. Key mechanisms behind vancomycin resistance include:
- Modification of Target Sites: Certain enterococcal strains alter their peptidoglycan precursor from D-Ala-D-Ala to D-Ala-D-Lac (or D-Ala-D-Ser), drastically reducing vancomycin’s binding affinity.
- Transposons and Gene Clusters: Resistance genes (e.g., vanA, vanB) can be transferred between bacterial species. This lateral gene transfer fosters the spread of resistant phenotypes between different strains or genera.
- Vancomycin-Intermediate Staphylococcus aureus (VISA): Some S. aureus strains develop thicker cell walls or altered cell wall metabolism, allowing them to partially evade vancomycin’s effect while retaining some susceptibility.
- Clinical Impact: Infections caused by VRE or VRSA require alternative therapies, including linezolid, daptomycin, or newer agents like tigecycline. These infections often pose greater treatment complexity and heightened health risks.
To curb rising resistance, clinicians pursue antibiotic stewardship, ensure appropriate dosing, and limit vancomycin use to cases where it is absolutely necessary or when alternative therapies are not merited.
Drug Interactions of Vancomycin
Due to its potential for nephrotoxicity, vancomycin shares notable drug interactions with other agents harmful to the kidneys or ears:
- Aminoglycosides: Co-administration is sometimes needed for synergy in endocarditis. However, both classes can cause nephrotoxicity and ototoxicity, so TDM and frequent kidney function tests are crucial.
- Loop Diuretics (e.g., furosemide): These can potentiate ototoxic risks and reduce intravascular volume, exacerbating renal toxicity.
- Nonsteroidal Anti-Inflammatory Drugs (NSAIDs): Can influence renal perfusion, potentially intensifying vancomycin-induced renal injury.
- Anesthetic Agents: Some intravenous anesthetics may provoke or worsen “red man syndrome” if administered with vancomycin too rapidly. Prolonging the infusion time or using prophylactic antihistamines helps reduce this issue.
Educating prescribing physicians and pharmacists on monitoring protocols and possible interactions helps sustain effective vancomycin therapy without incurring additional risks to the patient.
Future Directions and Research
Though vancomycin remains an instrumental antibiotic, evolving resistance patterns and the pursuit of safer therapies propel continued research:
- Novel Glycopeptides: Modifications of the vancomycin backbone (e.g., oritavancin, telavancin, dalbavancin) provide broader activity or improved pharmacokinetic profiles while retaining potent Gram-positive coverage.
- Drug Delivery Innovations: Liposomal formulations and other advanced drug carriers might reduce toxicity by selectively releasing the antibiotic at infection sites, limiting systemic exposure.
- Antibiotic Stewardship and Diagnostics: Faster microbiological diagnostic tools (such as polymerase chain reaction or next-generation sequencing) help identify bacterial pathogens earlier, optimizing targeted therapy and preventing the indiscriminate use of vancomycin.
- Combination Therapies: Research is exploring synergy between vancomycin and novel anti-staphylococcal agents to expedite bacterial clearance and reduce the risk of emergent resistance.
Continuous vigilance in the medical community about prescribing behaviors, prompt detection of resistant organisms, and adoption of new antibiotic guidelines will shape the next chapter of vancomycin’s legacy.
Practical Tips for Clinicians
• Assess Renal Function: Before vancomycin initiation, measure serum creatinine or eGFR. Adjust the dose according to changes in renal function.
• Consider AUC-Based Dosing: Engage your clinical pharmacy or use validated software to ensure your patients achieve an optimal AUC/MIC ratio without unnecessarily high troughs.
• Slow Infusions: To minimize “red man syndrome,” infuse each 1 g dose over at least 1 hour, increasing time proportionally for larger doses.
• Watch for Drug Interactions: Pay special attention to other nephrotoxic or ototoxic agents.
• Evaluate Infection Type: For MRSA and other serious infections, always confirm organism sensitivities. Consider synergy for complex cases (e.g., prosthetic valve endocarditis).
• Maintain Good Hydration: Adequate hydration can reduce the likelihood of kidney damage, especially in patients with borderline renal function or older adults.
Conclusion
Vancomycin’s storied history and enduring relevance make it a hallmark antibiotic in the fight against Gram-positive infections, particularly resistant strains like MRSA. By inhibiting cell wall synthesis at a critical juncture—binding to the D-Ala-D-Ala terminus of peptidoglycan precursors—vancomycin serves as an invaluable “last resort” for potentially life-threatening diseases. Its pharmacokinetic profile, characterized by poor oral absorption and renal excretion, underscores the necessity for intravenous administration in systemic infections and mandatory therapeutic drug monitoring to avoid nephrotoxicity and other adverse effects.
Over decades of clinical use, the stewardship of vancomycin has evolved to include integrated monitoring strategies, revised dosing guidelines, and the introduction of new glycopeptide analogs. Careful application of these principles helps maintain vancomycin’s efficacy in the face of emerging resistance. Meanwhile, research continues to refine vancomycin’s role, from exploring combination regimens to perfecting dosing protocols that align maximum bacterial killing with minimal toxicity. For healthcare professionals, a thorough understanding of vancomycin pharmacology is paramount as we strive to preserve this critical antibiotic for future generations.
Disclaimer: This article provides general information on vancomycin pharmacology and is not a substitute for professional medical advice. Individual patient care requires a personalized approach under the guidance of a qualified healthcare practitioner. Always consult with infectious disease specialists, pharmacists, and clinicians experienced in antibiotic stewardship before modifying or initiating vancomycin therapy.