Introduction
Rivaroxaban is an oral direct Factor Xa inhibitor widely used for the prevention and treatment of thromboembolic disorders. Developed as part of a newer generation of target-specific oral anticoagulants (TSOACs) or direct oral anticoagulants (DOACs), rivaroxaban offers several advantages over older agents such as warfarin and low-molecular-weight heparins. Marked by high specificity for Factor Xa, rivaroxaban effectively prevents conversion of prothrombin (Factor II) to thrombin (Factor IIa) in the coagulation cascade, conferring a potent anticoagulant effect (Goodman & Gilman, 2018).
By inhibiting free Factor Xa and Factor Xa bound in the prothrombinase complex, rivaroxaban exerts consistent thrombin-suppressive properties across diverse clinical settings, including atrial fibrillation and venous thromboembolism (VTE) prophylaxis or treatment. Approved by global regulatory agencies, rivaroxaban has been positioned as a convenient, once-daily regimen for many indications, eliminating the need for routine INR monitoring. However, the drug’s potential bleeding risk, interactions, and the partial availability of reversal agents mandate a thorough understanding of its pharmacodynamics, pharmacokinetics, clinical utility, and risk management.
This comprehensive review explores rivaroxaban’s mechanisms of action, pharmacological attributes, therapeutic indications, safety profile, drug interactions, and best practices. Drawing on foundational references including “Goodman & Gilman’s The Pharmacological Basis of Therapeutics” (13th Edition), “Katzung & Trevor’s Basic & Clinical Pharmacology” (14th Edition), and “Rang & Dale’s Pharmacology” (8th Edition), we highlight how rivaroxaban has reshaped anticoagulant therapy and continues to inform the evolution of coagulation pharmacology.
Chemistry and Mechanism of Action
Chemical Structure
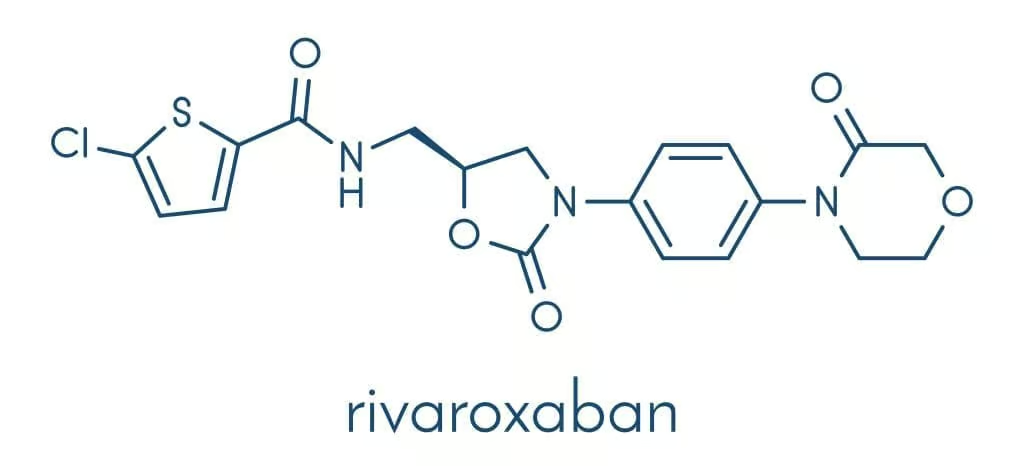
Rivaroxaban is a small-molecule anticoagulant belonging to the oxazolidinone class. Its structure features a morpholinone ring that confers high selectivity for Factor Xa. The compound was designed to bind with S1 and S4 pockets of Factor Xa’s active site, leading to its potent inhibitory action (Katzung, 2020).
Target and Mode of Action

Factor Xa serves as an essential enzyme in the final common coagulation pathway, converting prothrombin (Factor II) to thrombin (Factor IIa). Thrombin then promotes fibrinogen cleavage into fibrin monomers and activates platelets, culminating in fibrin-based clot formation. By selectively blocking this step, rivaroxaban substantially diminishes thrombin generation and thereby impedes clot development (Goodman & Gilman, 2018).
Unlike unfractionated heparin or low-molecular-weight heparins (LMWHs), which require antithrombin as a cofactor, rivaroxaban directly inhibits Factor Xa independent of antithrombin. This mechanism streamlines the anticoagulant response, minimizes inter-patient variability, and helps ensure predictable pharmacological action. Rivaroxaban inhibits both free Factor Xa and Factor Xa within the prothrombinase complex, though it does not require binding to platelet surfaces (Rang & Dale, 2019).
Pharmacodynamic Effects
The degree of Factor Xa inhibition correlates dose-dependently with reduced thrombin generation and decreased fibrin formation. Clotting assays, such as anti-Factor Xa levels, measure rivaroxaban’s anticoagulant intensity. Although routine therapeutic monitoring is not mandated, advanced or specialized lab tests (e.g., chromogenic anti-FXa assay) can quantify drug levels in special populations (Goodman & Gilman, 2018).
Pharmacokinetics
Absorption
Rivaroxaban has high oral bioavailability—roughly 80–100%—when administered at doses up to 10 mg once daily. Higher doses (15–20 mg) benefit from intake with food to optimize absorption. Food increases the bioavailability from around 66% (fasted) to nearly 100% (fed), an important detail particularly for prophylaxis or treatment regimens requiring higher daily doses (Katzung, 2020).
Distribution
After absorption, rivaroxaban is moderately bound (~90%) to plasma proteins, chiefly albumin. This relatively high protein binding indicates a modest volume of distribution (~50 L). Rivaroxaban readily permeates the vascular compartment but does not accumulate excessively in tissues due to its balanced physiochemical properties (Rang & Dale, 2019).
Metabolism
Rivaroxaban undergoes hepatic biotransformation mediated via CYP3A4, CYP2J2, and non-CYP pathways (such as hydrolysis). Up to about two-thirds of a rivaroxaban dose is metabolized, with 50% excreted renally as inactive metabolites and ~50% through fecal/biliary pathways. The remaining one-third is excreted unchanged by the kidney (Goodman & Gilman, 2018).
Elimination and Half-Life
The elimination half-life of rivaroxaban is approximately 5–9 hours in healthy adults, extending to ~11–13 hours in the elderly. Renal and hepatic function significantly affect clearance. Impaired renal excretion prolongs drug exposure, elevating bleeding risks if dosage adjustments are not made (Katzung, 2020).
Key Pharmacokinetic Takeaways
- Oral Dosing: Once or twice daily, dependent on indication.
- Food Requirement: Doses ≥15 mg require administration with meal(s) to heighten absorption.
- Renal Clearance: Supports a major route for parent drug excretion. Renal impairment may necessitate dosage modifications or cautionary use (Rang & Dale, 2019).
Clinical Indications
Stroke Prevention in Atrial Fibrillation
Non-valvular atrial fibrillation (NVAF) markedly increases the risk of cardioembolic stroke. Rivaroxaban reduces stroke and systemic embolic events in moderate- to high-risk patients by preventing clot formation in the left atrial appendage. It is an alternative to warfarin, offering predictable effects and removing the burden of routine INR checks (Goodman & Gilman, 2018).
Venous Thromboembolism (VTE) Treatment
Rivaroxaban is indicated for the management of acute deep vein thrombosis (DVT) and pulmonary embolism (PE), and for preventing recurrent VTE. Its streamlined “single-drug approach”—initiating and continuing therapy without bridging heparin—can simplify outpatient management of VTE (Katzung, 2020).
VTE Prophylaxis After Orthopedic Surgery
Approved for prophylaxis following total hip or total knee arthroplasty, rivaroxaban (at doses of 10 mg once daily) effectively prevents postsurgical DVT or PE, offering patients a convenient oral alternative to LMWH injections (Rang & Dale, 2019).
Secondary Prevention of Acute Coronary Syndrome
In combination with antiplatelet therapy, low-dose rivaroxaban has been explored to reduce ischemic events in patients with recent acute coronary syndrome (ACS). Some guidelines incorporate rivaroxaban in select high-risk ACS or chronic ‘secondary prevention’ contexts (Goodman & Gilman, 2018).
Extended VTE Prophylaxis in Medically Ill Patients
Rivaroxaban has also been evaluated for extended prophylaxis in medically ill but stable patients with restricted mobility. This indication is less widely adopted but recognized in certain guidelines after risk-benefit evaluation (Katzung, 2020).
Dosing and Administration
Non-Valvular Atrial Fibrillation
Typical recommended dosing is 20 mg once daily with the evening meal in patients with CrCl >50 mL/min. In moderate renal impairment (CrCl 15–50 mL/min), a reduced dose (15 mg once daily with food) is often advised. Monitoring renal function is crucial to sustain safe use (Rang & Dale, 2019).
Treatment of DVT/PE
For acute VTE treatment:
- 15 mg twice daily with food for the initial 3 weeks, followed by 20 mg once daily with food.
In patients with moderate renal impairment, caution is warranted, with potential dose adjustments depending on regulatory guidance and clinical judgment (Goodman & Gilman, 2018).
VTE Prophylaxis After Orthopedic Surgery
- 10 mg once daily, starting 6–10 hours post-operative if hemostasis is secure. Duration typically extends 10–14 days for knee replacement and up to 35 days for hip replacement.
No routine anti-Xa monitoring is needed, though renal function and bleeding signs require clinical attention (Katzung, 2020).
Missed Dose Management
When a dose is missed:
- In once-daily regimens, patients should take the missed dose immediately and resume the next dose as scheduled.
- For twice-daily regimens (like in the initial 3-week VTE therapy phase), a patient can take two tablets at once to ensure the daily total of 30 mg is still achieved (Rang & Dale, 2019).
Efficacy and Comparative Trials
ROCKET AF (Rivaroxaban Once-daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Systemic Embolism Trial in Atrial Fibrillation)
In patients with non-valvular AF and moderate to high stroke risk, rivaroxaban was non-inferior to warfarin for stroke/systemic embolism prevention. Major bleeding rates were comparable, though rivaroxaban showed lower incidence of intracranial hemorrhage (Goodman & Gilman, 2018).
EINSTEIN-DVT and EINSTEIN-PE
These trials demonstrated rivaroxaban’s non-inferiority compared to enoxaparin/vitamin K antagonist bridging for treating acute DVT and PE, highlighting fewer bleeding complications and simpler outpatient management. Rivaroxaban’s single-drug approach proved effective for preventing VTE recurrence (Katzung, 2020).
RECORD Trials (Regulation of Coagulation in Major Orthopaedic Surgery Reducing the Risk of DVT and PE)
In postoperative prophylaxis after total knee or hip replacement, rivaroxaban (10 mg daily) was superior to enoxaparin in reducing VTE events. Bleeding risk was slightly elevated but generally manageable (Rang & Dale, 2019).
COMPASS Trial
Evaluated low-dose rivaroxaban (2.5 mg twice daily) plus aspirin in stable atherosclerotic vascular disease. Combined therapy reduced the risk of cardiovascular events compared to aspirin alone, albeit with a higher bleeding incidence. This formed the basis for certain guidelines endorsing combined antithrombotic strategies in high-risk patients (Goodman & Gilman, 2018).
Adverse Effects
Bleeding
As with all anticoagulants, hemorrhage remains the principal concern. Clinically significant bleeding can manifest as gastrointestinal bleeding, hematuria, epistaxis, or in severe cases, intracranial hemorrhage. The risk is dose-dependent and influenced by patient factors (e.g., renal function, concurrent medications, advanced age).
Gastrointestinal Complaints
Dyspepsia, nausea, or abdominal discomfort can occur, although these are generally mild compared to warfarin or dabigatran. Taking rivaroxaban with food can help mitigate GI side effects for the higher doses (Katzung, 2020).
Elevated Liver Enzymes
A mild transient rise in hepatic transaminases was noted in certain trial participants, though severe hepatotoxicity is rare. Monitoring LFTs in patients with existing hepatic disease or risk factors is prudent (Rang & Dale, 2019).
Hypersensitivity
Rarely, rash or allergic reactions can occur. Discontinuation is indicated if severe hypersensitivity or anaphylaxis arises.
Reversal and Management of Bleeding
Andexanet Alfa
A specific reversal agent, andexanet alfa, is approved for factor Xa inhibitors (including rivaroxaban). Andexanet alfa binds direct Factor Xa inhibitors with high affinity, neutralizing their effect. However, cost and availability can be limiting factors (Goodman & Gilman, 2018).
Prothrombin Complex Concentrate (PCC)
For major bleeding when andexanet is unavailable, PCC (4-factor) can help restore clotting factor levels. Though not a direct antidote, PCC can partially reverse rivaroxaban’s anticoagulant effect by replenishing vitamin K–dependent factors (II, VII, IX, X). Thromboelastography or anti-FXa levels may guide re-dosing (Katzung, 2020).
Supportive Measures
- Activated charcoal if ingestion occurred recently (<2 hours).
- Hemodynamic support (IV fluids, pressors if needed).
- Local measures to control bleeding.
- Monitoring hemoglobin, hematocrit, and coagulation parameters carefully (Rang & Dale, 2019).
Drug Interactions
P-glycoprotein and CYP3A4 Substrates
Rivaroxaban is a substrate for P-glycoprotein (P-gp) and is extensively metabolized by CYP3A4. Co-administration with strong dual inhibitors of CYP3A4 and P-gp (e.g., ketoconazole, ritonavir) can raise rivaroxaban plasma concentrations, escalating bleeding risk. Conversely, potent inducers (e.g., rifampin, carbamazepine, phenytoin) may reduce rivaroxaban levels, risking thrombosis (Goodman & Gilman, 2018).
Pharmacodynamic Interactions
Simultaneous use with other anticoagulants (e.g., heparin, warfarin) or antiplatelets (aspirin, P2Y12 inhibitors, NSAIDs) compound bleeding risk. Clinical rationale and vigilant monitoring for hemorrhagic events are essential if partial combination therapy is justified (Katzung, 2020).
Renal and Hepatic Impairment
- Renal Dysfunction: Rivaroxaban clearance diminishes, potentially elevating blood levels. Dose reductions or avoidance may be required in severe impairment (CrCl <15 mL/min).
- Hepatic Disease: In moderate or severe hepatic impairment, rivaroxaban use is typically contraindicated or approached with extreme caution, given unpredictable metabolism (Rang & Dale, 2019).
Special Populations
Elderly Patients
Due to decreased renal function and potential polypharmacy, older adults may exhibit heightened rivaroxaban exposure and bleeding risk. Dose adjustments and frequent assessment of renal function are recommended (Goodman & Gilman, 2018).
Pediatric Use
While rivaroxaban has garnered approvals for certain pediatric indications (like VTE prophylaxis in children post-Fontan procedure or after the appropriate studies), it remains an area of ongoing research. Pediatric dosing is weight-based, with careful monitoring for side effects (Katzung, 2020).
Pregnancy and Lactation
Direct Factor Xa inhibitors generally lack robust safety data in pregnant patients, and concerns about placental transport and hemorrhage risk hamper usage. Low-molecular-weight heparin is usually preferred in pregnancy. Rivaroxaban is not recommended during breastfeeding due to probable excretion in breast milk (Rang & Dale, 2019).
Extreme Obesity
In patients with BMI >40 kg/m² or weight >120 kg, limited data exist on rivaroxaban’s pharmacokinetics. Some guidelines recommend caution or additional anti-FXa monitoring to confirm adequate anticoagulation (Goodman & Gilman, 2018).
Advantages over Vitamin K Antagonists
- Predictable Pharmacokinetics: Minimizes the need for routine INR monitoring.
- Rapid Onset: Therapeutic anticoagulation is reached within hours of the first dose, eliminating bridging heparin in many cases.
- Fewer Food Interactions: Unlike warfarin, rivaroxaban has minimal dietary constraints, except that higher doses should be taken with food.
- Fixed Dosing: No requirement for continuous dose adjustments based on lab tests (Katzung, 2020).
However, cost considerations, lack of a fully established universal monitoring parameter, residual bleeding risk, and certain drug interactions remain important limitations.
Limitations and Criticisms
- Bleeding Risk: Despite having possibly lower intracranial hemorrhage risk than warfarin, GI bleeding can be more frequent at higher doses, particularly in older individuals.
- Renal Dependence: Suboptimal for those with advanced renal failure.
- Lack of Monitoring Tools: No commonly available routine test similar to INR for real-time adjustments, although anti-FXa assays exist in some tertiary centers.
- Reversal Agents: While andexanet alfa is nominally effective, cost and accessibility constraints hamper its broad usage (Rang & Dale, 2019).
Evidence from large-scale trials has established rivaroxaban’s place, but real-world data collection continues to refine best practices and usage guidelines.
Practical Considerations and Monitoring
Patient Selection
- Non-valvular AF: Evaluate stroke risk (e.g., CHA₂DS₂-VASc score) and bleeding risk (e.g., HAS-BLED) to weigh rivaroxaban’s net benefit.
- VTE: Rivaroxaban suits patients compliant with daily oral therapy and lacking major renal or hepatic compromise. It avoids bridging complexities.
- Postoperative Prophylaxis: Orthopedic procedures with moderate-high VTE risk.
- Secondary Prevention: Some complex ACS or peripheral artery disease cases after weighing bleeding risk (Goodman & Gilman, 2018).
Laboratory Assessment
- Renal Function: eGFR or CrCl at baseline and periodically is vital.
- Hepatic Panel: Baseline LFTs in patients with suspected or known liver disease.
- Bleeding Indices: CBC, hemoglobin, hematocrit for subtle anemia detection.
While routine rivaroxaban lab measurement (anti-FXa) is not standard, it can be performed in special situations (like major bleeding, extreme body weight, or prior to emergent surgery) (Katzung, 2020).
Patient Education
- Drug Adherence: Missing doses at the higher-end regimens can compromise anticoagulation efficacy.
- Signs of Bleeding: Encourage immediate reporting of hematuria, melena, or unusual bruising.
- Medication & Food Interactions: Clarify avoidance of concurrent strong CYP3A4/P-gp inducers/inhibitors without prescriber knowledge.
- Surgery or Procedure: Rivaroxaban typically requires temporary discontinuation 24–48 hours prior to invasive procedures, depending on bleeding risk (Rang & Dale, 2019).
Emerging Data and Future Directions
Real-World Evidence
Expanding registry analyses and observational studies continue to confirm rivaroxaban’s robust performance for stroke prevention and VTE prophylaxis. Studies clarify bleeding patterns, risk factors, and clinical outcomes across practice settings beyond initial pivotal trials (Goodman & Gilman, 2018).
Extended Indications
Investigations focus on rivaroxaban’s role in other prothrombotic conditions, including cancer-associated thrombosis (though low-molecular-weight heparin remains a mainstay). Some data propose bridging strategies for mechanical heart valves, though DOACs are not generally recommended for mechanical valve recipients at present (Katzung, 2020).
Biomarker-Guided Dosing
As personalized medicine evolves, validating a simple, clinically feasible anti-FXa assay or exploring genetic markers influencing rivaroxaban metabolism may refine therapy in borderline cases (Rang & Dale, 2019).
Combined Therapy Approaches
Dual pathway inhibition (DPI) merges low-dose rivaroxaban with antiplatelet therapy for stable coronary or peripheral arterial disease. Ongoing research and guidelines refine which patient subsets gain net benefit from such regimens amid increased bleeding risk (Goodman & Gilman, 2018).
Clinical Pearls
- Once-Daily in AF: Rivaroxaban 20 mg daily (or 15 mg if moderate renal impairment) simplifies stroke prevention in non-valvular AF. Confirm creatinine clearance first.
- Food is Key: Doses of 15 or 20 mg must be taken with meals to ensure full absorption; the 10 mg prophylactic dose does not strictly require food.
- Major Bleeding Management: Andexanet alfa is a specific antidote if accessible. Otherwise, 4-factor PCC is recommended in life-threatening hemorrhage.
- Medication Coordination: Watch out for potent CYP3A4/P-gp inducers (phenytoin, rifampin) or inhibitors (ketoconazole, ritonavir).
- Bridging: Typically not required in rivaroxaban usage for VTE, as the drug quickly attains therapeutic levels.
- Kidney Health: Check eGFR frequently, especially in older adults. Potential dose adjustments or therapy change required if eGFR <30 mL/min/1.73m².
- Procedural Interruptions: For surgeries with low bleeding risk, cessation typically 24 hours prior may suffice; for moderate-high bleeding risk, at least 48 hours or more, guided by physician judgment (Katzung, 2020).
Conclusion
Rivaroxaban has redefined the landscape of anticoagulant therapy by coupling direct Factor Xa inhibition with convenient oral dosing, predictable pharmacodynamics, and broad efficacy across atrial fibrillation, venous thromboembolism, and certain subsets of coronary artery disease. Clinical trials (e.g., ROCKET AF, EINSTEIN, RECORD) validated its non-inferiority or superiority versus warfarin and enoxaparin, prompting widespread adoption (Goodman & Gilman, 2018).
In unleashing this potent “one-size-fits-all” approach for multiple indications, rivaroxaban addresses many limitations of traditional anticoagulants—no frequent INR checks, fewer drug-food constraints, and rapid onset/offset. That said, bleeding complications, especially gastrointestinal, remain a consideration. Additionally, caution is paramount in patients with advanced renal disease, hepatic dysfunction, or potent drug interactions. The partial introduction of andexanet alfa as a reversal agent has somewhat mitigated hemorrhagic concerns, though cost and access issues persist (Katzung, 2020).
Looking forward, rivaroxaban’s role in combination therapy, its application to new prophylactic or therapeutic niches, and personalized dosing strategies remain dynamic areas of investigation. For healthcare professionals, a solid grasp of rivaroxaban’s pharmacology, safety parameters, and best-practice dosing across indications is vital. Balancing robust anticoagulation versus bleeding risk, guided by evidence-based protocols, underscores rivaroxaban’s continued prominence in modern coagulation management (Rang & Dale, 2019).
References (Book Citations)
- Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 13th Edition.
- Katzung BG, Basic & Clinical Pharmacology, 14th Edition.
- Rang HP, Dale MM, Rang & Dale’s Pharmacology, 8th Edition.