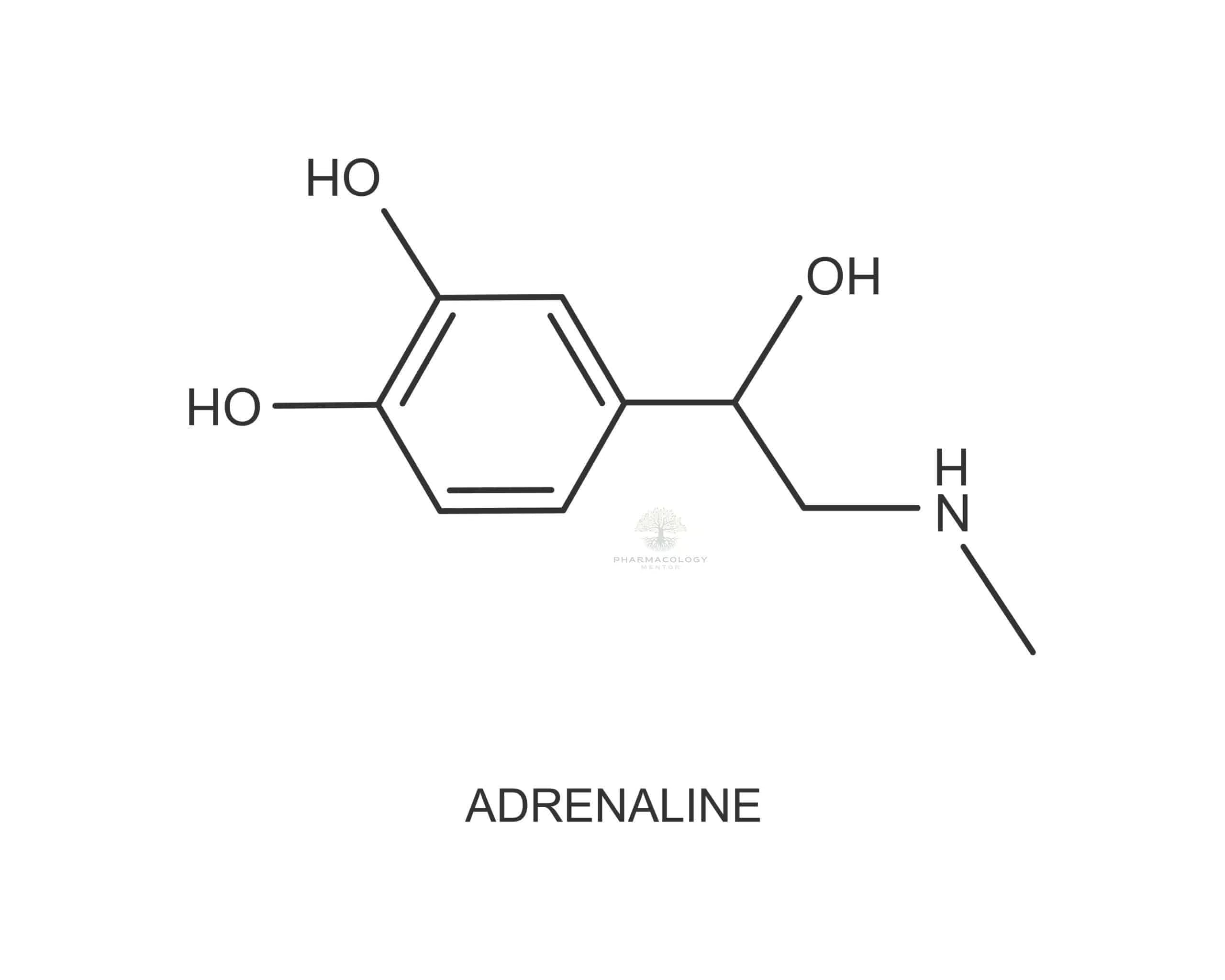
Adrenaline, also referred to by its international nonproprietary name epinephrine, is a naturally occurring catecholamine released primarily by the adrenal medulla. As one of the most crucial hormones in the human body’s “fight-or-flight” response, adrenaline orchestrates a range of physiological changes to prepare the body for stress or danger. In medicine, synthetic or pharmaceutical forms of adrenaline are used to treat an array of conditions, including anaphylaxis, cardiac arrest, and asthma exacerbations. This SEO-optimized article presents a comprehensive overview of the pharmacology of adrenaline, delving into its historical background, mechanism of action, pharmacodynamics, clinical uses, side effects, and future directions. By exploring these major facets, clinicians, pharmacology students, and healthcare enthusiasts will gain a solid understanding of how adrenaline shapes both normal physiology and indispensable therapeutic interventions.
Historical Background of Adrenaline
The discovery and naming of adrenaline (epinephrine) date back to the late 19th and early 20th centuries. In 1895, Polish physiologist Napoleon Cybulski first identified an active principle released by the adrenal glands. However, the name “adrenaline” was proposed by Japanese chemist Jokichi Takamine in the early 1900s when he successfully isolated the active agent from the adrenal glands. Meanwhile, American pharmacologist John Abel, working independently, crystallized an adrenal extract he called “epinephrin,” which eventually became the universal term for the hormone in the United States (epinephrine).
Throughout the 20th century, scientists uncovered adrenaline’s profound influence on cardiovascular and respiratory systems, paving the way for modern therapeutic uses. Today, it’s hard to imagine emergency medicine without adrenaline, especially in acute conditions like anaphylaxis or cardiac arrest. With a better understanding of adrenoceptors (adrenergic receptors), researchers developed synthetic derivatives and refined clinical applications, making adrenaline a bedrock of acute care management and a foundational concept in modern physiology.
Classification of Adrenaline
Biochemically, adrenaline (C₉H₁₃NO₃) belongs to the family of catecholamines, characterized by the presence of a catechol group (a benzene ring with two hydroxyl groups) and an amine group. This categorization aligns adrenaline with other major endogenous catecholamines such as noradrenaline (norepinephrine) and dopamine.
Adrenaline can also be viewed through the lens of its receptor pharmacology, primarily targeting two main classes of adrenergic receptors: alpha (α) and beta (β). Within these categories lie subtypes:
• Alpha Receptors: α₁ and α₂
• Beta Receptors: β₁, β₂, and β₃Adrenaline’s interactions with these receptors confer its wide-reaching physiological and therapeutic implications. Hence, understanding receptor selectivity plays a pivotal role in optimizing adrenaline’s clinical applications.
Biosynthesis and Release of Adrenaline
In the human body, the adrenal medulla produces adrenaline through a regulated biosynthetic pathway:
- Tyrosine Uptake: The amino acid tyrosine (from dietary sources or synthesized in the liver) enters chromaffin cells in the adrenal medulla.
- DOPA Formation: Tyrosine is hydroxylated to dihydroxyphenylalanine (DOPA) via the enzyme tyrosine hydroxylase.
- Dopamine Synthesis: DOPA is decarboxylated into dopamine.
- Noradrenaline Production: Dopamine is transported into storage vesicles and then converted to noradrenaline (norepinephrine) by dopamine β-hydroxylase.
- Adrenaline Formation: Finally, phenylethanolamine N-methyltransferase (PNMT) in the cytosol methylates noradrenaline to form adrenaline, which is then taken back into chromaffin granules for storage until release.
Physiological triggers—such as stress, low blood glucose, or sympathetic nervous system activation—prompt an influx of calcium ions into chromaffin cells, thus facilitating exocytosis of adrenaline stored in vesicles. Once released into the bloodstream, adrenaline exerts diverse actions via its receptors on target tissues throughout the body.
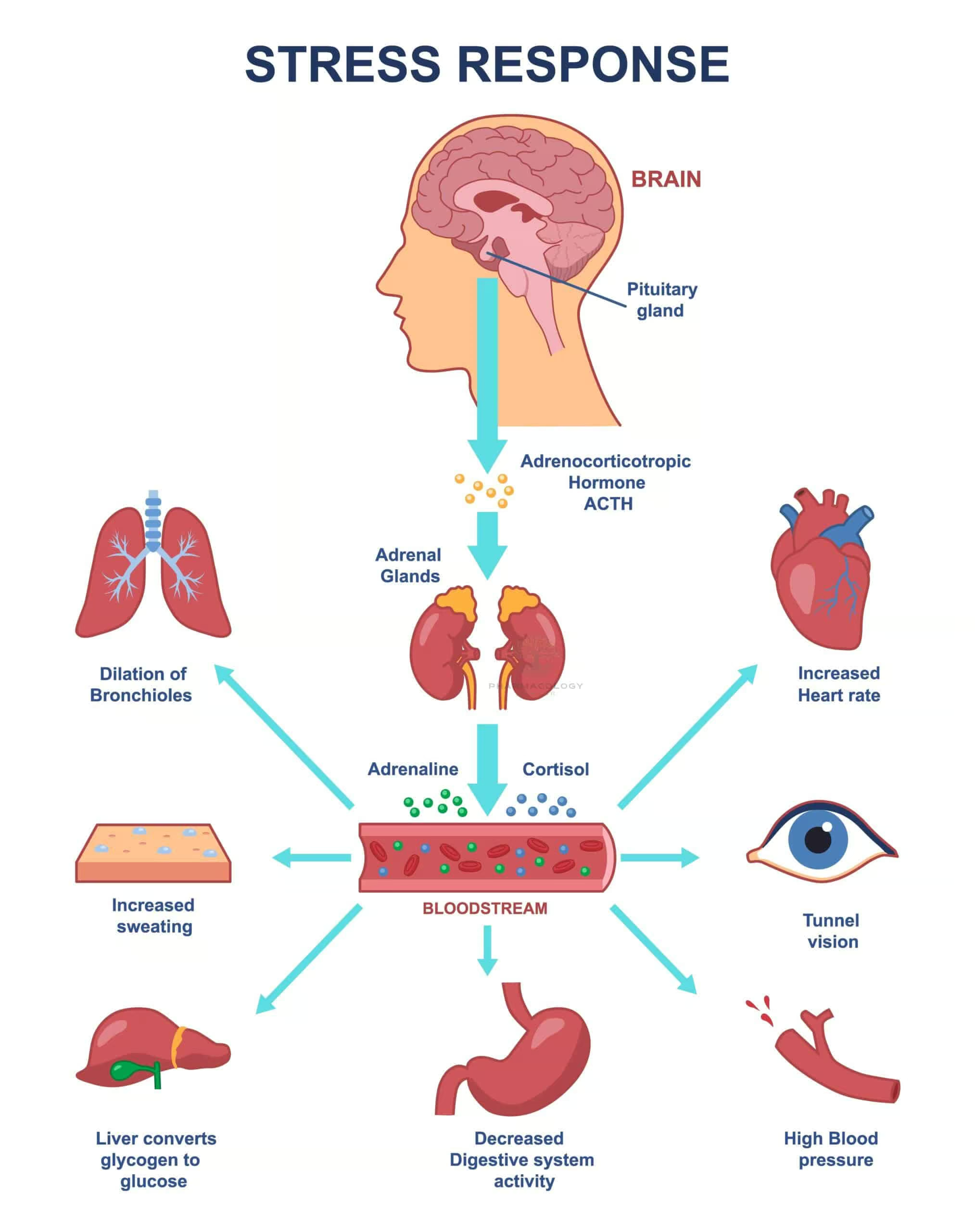
Mechanism of Action of Adrenaline
Adrenaline mediates its physiological and pharmacological effects through G protein-coupled receptors (GPCRs) known as adrenergic receptors. When adrenaline binds to these receptors, various intracellular signaling cascades ensue:
- Alpha-1 (α₁) Receptors: Couples typically to Gq proteins, stimulating the phospholipase C pathway. This leads to increased inositol triphosphate (IP₃) and diacylglycerol (DAG), promoting smooth muscle contraction and vasoconstriction in certain vascular beds.
- Alpha-2 (α₂) Receptors: Often linked to Gi proteins, inhibiting adenylate cyclase. This lowers cyclic AMP (cAMP) levels, causing inhibitory effects such as decreased sympathetic outflow, reduced insulin secretion, and reduced presynaptic noradrenaline release.
- Beta-1 (β₁) Receptors: Primarily found in the heart, coupling to Gs proteins. When activated, they enhance adenylate cyclase activity, raising cAMP. This results in increased heart rate (positive chronotropy), enhanced contractility (positive inotropy), and faster AV node conduction (positive dromotropy).
- Beta-2 (β₂) Receptors: Also linked to Gs proteins, prevalent in smooth muscle of the bronchi, vasculature in skeletal muscle, and the uterus. Activation raises cAMP and typically facilitates bronchodilation, vasodilation, and uterine relaxation.
- Beta-3 (β₃) Receptors: Though less relevant in acute clinical settings, β₃ receptors stimulate lipolysis in adipose tissue under certain conditions.
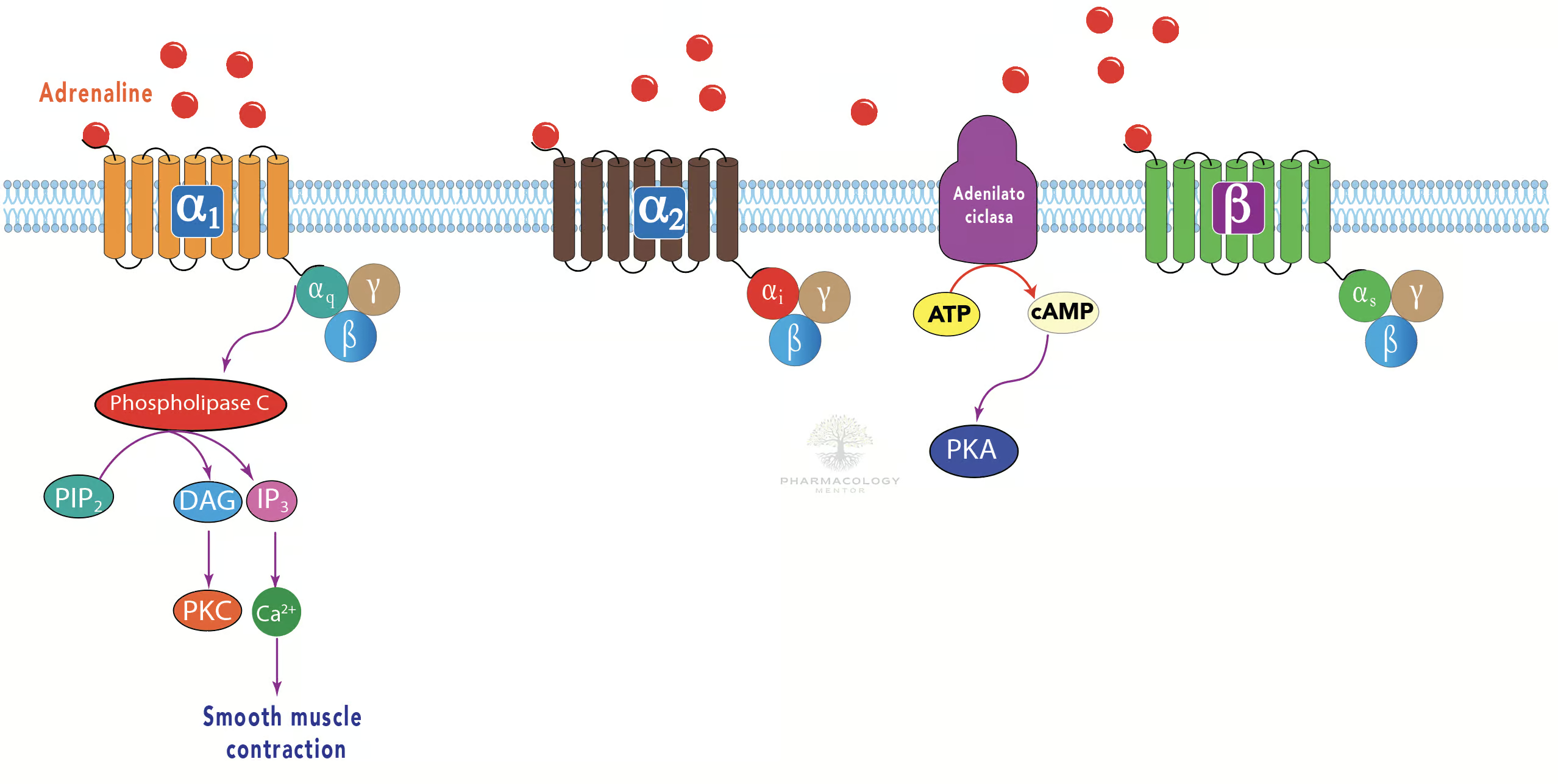
The net physiological outcome depends on the relative density and affinity of adrenaline for these receptor subtypes in a given tissue, and on the overall local concentration of the hormone. This complexity allows for a highly regulated system that balances bodily demands during stressful situations.
Pharmacodynamics
By binding to multiple adrenergic receptor subtypes, adrenaline exerts a pleiotropic set of physiological responses critical for the fight-or-flight reaction:
• Cardiovascular Effects:
– Increased Heart Rate (β₁ effect)
– Enhanced Contractility (β₁ effect)
– Vasoconstriction in Certain Beds (α₁ effect)
– Vasodilation in Skeletal Muscle (β₂ effect)
• Respiratory Effects:
– Bronchodilation (β₂ effect)
– Decreased release of inflammatory mediators from mast cells (β₂ effect)
• Metabolic Changes:
– Glycogenolysis and Gluconeogenesis in the liver (β₂ effect)
– Lipolysis in adipose tissue (β₃ effect)
– Reduced insulin secretion (α₂ effect) and increased glucagon secretion (β₂ effect)
• Other Effects:
– Pupillary dilation (via α₁ stimulation in iris dilator muscle)
– Sweating and piloerection (though these involve additional sympathetic co-transmitters)These concerted actions ensure the body can rapidly mobilize energy stores, elevate cardiac output, and direct blood flow to critical organs (heart, brain, skeletal muscle) during acute stress. Clinically, understanding these diverse constructs aids in employing adrenaline in emergency interventions and anticipating side effects.
Pharmacokinetics
In medical contexts, adrenaline is typically administered parenterally (intravenous, intramuscular, or subcutaneous routes). Key pharmacokinetic considerations include:
- Absorption:
– Intramuscular (IM) injection in the mid-outer thigh (e.g., via an auto-injector such as the EpiPen) leads to fast and relatively reliable absorption.
– Intravenous (IV) injection has an immediate onset but mandates close monitoring to avoid excessive plasma concentrations.
– Subcutaneous (SC) administration can yield slower absorption due to local vasoconstriction, although heat or massage may increase uptake. - Distribution:
– Adrenaline is widely distributed, crossing some tissue barriers but generally not entering the central nervous system in significant amounts under typical circumstances.
– Plasma protein binding is variable and considered relatively low. - Metabolism:
– Primarily metabolized by catechol-O-methyltransferase (COMT) and monoamine oxidase (MAO) in the liver and other tissues, forming inactive metabolites like metanephrine and vanillylmandelic acid (VMA). - Elimination:
– Urinary excretion of metabolites is the main route of elimination, typically within 24 hours.
– Adrenaline has a short half-life, usually only about 2-3 minutes in circulation, although physiological effects can outlast these direct levels due to downstream signaling sequences. - Considerations:
– In shock states (e.g., anaphylactic shock), peripheral perfusion may be compromised, altering drug absorption.
– Continuous IV infusions can maintain stable plasma levels, essential for hemodynamic support in advanced life support or ICU settings.
Mastering adrenaline’s pharmacokinetics adds precision to how and when clinicians deploy the agent, striking a balance between immediate efficacy and the risk of complications.
Clinical Uses of Adrenaline
Adrenaline’s remarkable capacity to modulate cardiovascular and respiratory function underpins multiple therapeutic applications:
- Anaphylaxis:
– Intramuscular injection of adrenaline is the first-line treatment for life-threatening allergic reactions. By reversing bronchospasm (β₂) and mitigating vasodilation/peripheral edema (α₁), adrenaline often saves lives in acute anaphylaxis.
– Repeated doses may be necessary if symptoms persist or recur. - Cardiac Arrest:
– In advanced cardiac life support (ACLS) protocols, IV/IO adrenaline boluses are administered to boost coronory perfusion pressure and augment the likelihood of return of spontaneous circulation (ROSC).
– The recommended dose in adults is typically 1 mg IV adrenaline every 3-5 minutes during a cardiac arrest scenario. - Acute Asthma or Bronchospasm:
– Historically, subcutaneous or intramuscular adrenaline injections were used for severe asthma attacks, exploiting β₂-mediated bronchodilation.
– While alternatives like inhaled β₂ agonists (e.g., salbutamol) are often preferred, adrenaline still provides significant emergency relief when rescue inhalers fail or in anaphylactic asthma. - Local Vasoconstriction:
– Often adrenaline is added in small amounts to local anesthetic solutions (e.g., lidocaine with adrenaline) to prolong anesthesia by shrinking local blood vessels (α₁ effect), reducing systemic absorption of the anesthetic, and controlling bleeding in surgical sites. - Shock and Hypotension:
– In hypoperfusion states (e.g., septic shock) refractory to volume resuscitation and first-line vasopressors like noradrenaline, adrenaline can bolster blood pressure by its α₁ and β₁ actions.
– Careful infusion titration is vital due to the risk of arrhythmias and excessive vasoconstriction. - Glaucoma (Rare/Older Use):
– Before more targeted therapies, topical adrenaline or dipivefrin (an adrenaline prodrug) were used to lower intraocular pressure. This approach is less common now given newer medications with fewer systemic side effects.
Such broad indications make adrenaline an indispensable element in emergency and critical care. However, training and awareness of potential hazards are crucial to maximize therapeutic benefits.
Adverse Effects and Toxicities
An overabundance of adrenergic stimulation from adrenaline can produce a variety of side effects, generally correlating with its robust receptor agonism:
- Cardiovascular Complications:
– Tachycardia, palpitations, and arrhythmias such as supraventricular tachycardia or ventricular fibrillation can occur.
– Hypertension (particularly systolic) may precipitate hemorrhagic events like cerebral hemorrhage in susceptible individuals. - Central Nervous System (CNS):
– Anxiety, tremor, restlessness, and headache frequently manifest due to heightened sympathetic drive.
– Large doses may provoke acute panic symptoms or severe agitation. - Metabolic Disturbances:
– Hyperglycemia can surge due to glycogenolysis and gluconeogenesis, potentially complicating glycemic control in diabetic patients.
– Elevated free fatty acids as a result of lipolysis (β₃ effect). - Skin and Extremities:
– Pallor (from vasoconstriction), cold extremities, and possible tissue necrosis at injection sites if infiltration occurs outside blood vessels or if used in end-arterial fields (e.g., digits, nose, penis). - Shift in Potassium:
– Stimulation of β₂ receptors may drive potassium into cells, occasionally causing transient hypokalemia, which can provoke muscle weakness or conduction anomalies.
Clinicians must remain vigilant when administering adrenaline, especially in patients with pre-existing cardiovascular disease or uncontrolled hyperthyroidism, as they tend to be more susceptible to adrenaline’s adverse effects.
Contraindications and Cautions
While adrenaline can be lifesaving in emergencies, certain entities demand caution:
- Absolute Contraindications:
– There are few absolute contraindications when adrenaline is urgently required (e.g., in anaphylaxis or cardiac arrest), as the risk of not giving adrenaline can be fatal. - Relative Contraindications:
– Patients with narrow-angle glaucoma: Pupillary dilation may worsen intraocular pressure.
– Uncontrolled hyperthyroidism: Heightened risk of arrhythmias and hypertensive crisis.
– Severe hypertension or coronary artery disease: Potential for myocardial ischemia or stroke.
– Beta-blocker therapy: Can lead to paradoxical hypertension if β₂-mediated vasodilation is blocked, leaving unopposed α₁ constriction.
– Halogenated anesthetics: Adrenaline increases arrhythmogenic risk when combined with some inhalation anesthetics, particularly halothane.
Given adrenaline’s potency, risk-benefit evaluations drive conscious use, ensuring that in genuine emergencies, the life-saving potential predominates over theoretical hazards.
Special Considerations for Different Populations
- Pediatric Patients:
– Dosing for anaphylaxis or resuscitation in children follows weight-based guidelines, typically 0.01 mg/kg (up to 0.3 mg or 0.5 mg) for an anaphylaxis auto-injector.
– Pediatric patients can be more prone to systemic side effects if dosing is not precisely adjusted, emphasizing the need for accurate weight measurements and standardized protocols. - Pregnant Patients:
– Adrenaline crosses the placenta, and maternal hypertension or uterine vasoconstriction could diminish fetal blood flow. However, it may be needed for anaphylaxis or cardiac arrest even in pregnant women, as maternal survival is paramount.
– Cautious administration is advised in postpartum hemorrhage or while on certain obstetric medications. - Elderly Individuals:
– More likely to have coronary artery disease, arrhythmias, or compromised renal function, so smaller doses or gentler titration might be required.
– Close ECG monitoring ensures timely identification of potentially dangerous arrhythmias. - Patients with Comorbidities:
– Diabetes mellitus: Potential hyperglycemia.
– Thyroid disorders: Risk of arrhythmias, especially in hyperthyroidism.
– Pheochromocytoma: Excess endogenous catecholamines might interact unpredictably with exogenous adrenaline administration.
Tailoring adrenaline therapy to suit each individual’s unique physiology is a hallmark of prudent clinical practice.
Drug Interactions
Because adrenaline exerts potent effects across multiple organ systems, it exhibits notable interactions with other medications:
- Beta-Blockers:
– Nonselective beta-blockers (e.g., propranolol) can lead to unopposed α₁ vasoconstriction, causing severe hypertension and possible reflex bradycardia.
– Cardioselective β₁-blockers (e.g., metoprolol) mitigate this risk, though caution remains prudent. - Tricyclic Antidepressants and Monoamine Oxidase Inhibitors (MAOIs):
– These agents could inhibit the metabolism or uptake of catecholamines, amplifying adrenaline’s effects, sometimes dangerously. - Inhalation Anesthetics:
– Some agents (e.g., halothane) sensitize the myocardium to catecholamines, heightening arrhythmogenic risk. - Alpha-Adrenergic Blockers:
– Drugs like phentolamine can counteract adrenaline’s vasoconstrictive α₁ effect, often leading to decreased blood pressure if used concurrently. - Insulin and Oral Hypoglycemics:
– Adrenaline-induced hyperglycemia can diminish the efficacy of antidiabetic therapies, necessitating close glucose monitoring.
Maintaining an updated medication record and checking references or local hospital formulary guidelines is vital to avoid hazardous drug interactions.
Overdose Management
Excess adrenaline can cause hypertensive crisis, tachyarrhythmias, and even pulmonary edema. Management strategies focus on symptomatic and supportive care:
- Medication Discontinuation: Immediately stop or reduce infusion if adrenaline was being administered IV.
- Alpha-Blockers: Agents like phentolamine can help manage severe hypertensive episodes caused by excessive vasoconstriction.
- Beta-Blockers for Arrhythmias: In controlled circumstances, short-acting beta-1 blockers (e.g., esmolol) may suppress life-threatening tachycardias, though the risk of unopposed α effects demands vigilance.
- Benzodiazepines: May calm CNS agitation or reduce tremors and anxiety in mild overdoses.
- Airway and Hemodynamic Support: Oxygen supplementation, intravenous fluids, and mechanical ventilation if needed when pulmonary edema or severe cardiopulmonary instability is present.
Early recognition and prompt action decisively reduce morbidity and mortality in adrenaline overdose.
Future Directions in Adrenaline Research
Despite adrenaline’s long-standing place in acute medicine, research explores how best to optimize its benefits while curbing risk:
- Pharmaceutical Formulations: Improved auto-injector designs and stable adrenaline formulations are under constant development to facilitate ease of use and longevity.
- Alternative Administration Routes: Nasal adrenaline sprays or inhaled nebulization, for certain emergencies, aim to boost accessibility and minimize systemic side effects.
- Adjunctive Agents: Investigations continue into combining adrenaline with new molecules to prolong action in local anesthesia or to refine anaphylaxis management.
- Precision Dosing for Cardiac Arrest: Increasingly, the one-size-fits-all approach in advanced cardiac life support is being questioned, with some evidence suggesting that personalized adrenaline dosing or approaches like delayed administration in specific arrhythmias could improve outcomes.
- Pharmacogenomics: Variation in adrenergic receptor genes may explain different patient responses; as pharmacogenomics evolves, tailored regimens could become standard care.
By harnessing these findings, clinicians and researchers strive to refine adrenaline’s role—making it safer, more selective, and, ultimately, more effective in life-threatening scenarios.
Practical Pearls for Clinicians
• Use IM Injection in Anaphylaxis: Recommend the lateral thigh site (mid-outer thigh) for rapid and reliable absorption.
• IV Administration Requires Monitoring: When given IV for cardiac arrest or severe hypotension, continuous ECG, blood pressure, and possibly invasive arterial lines for real-time feedback are crucial.
• Anticipate Tachyarrhythmias: Preexisting arrhythmic tendencies can worsen with adrenaline. Have antiarrhythmic measures ready if needed.
• Counsel Patients on Auto-Injectors: Ensure they understand correct usage, storage conditions, and the necessity of emergency follow-up after an adrenaline injection.
• Monitor Blood Glucose in Diabetics: Adjust therapy or increased glucose monitoring might be necessary due to adrenaline-driven hyperglycemia.
• Beware Beta-Blockers: The synergy between nonselective beta-blockade and exogenous adrenaline can produce dangerous hypertension.Balancing adrenaline’s life-saving capacity with a solid grasp of its complexities remains a cornerstone of acute and critical care practice.
Conclusion
From its historic discovery in adrenal extracts to its current standing as a critical lifeline in emergencies such as anaphylaxis and cardiac arrest, adrenaline (epinephrine) has significantly influenced the course of medicine. Central to adrenaline’s therapeutic versatility is its affinity for α- and β-adrenergic receptors, orchestrating a sophisticated range of cardiovascular, respiratory, and metabolic responses designed for immediate survival in threatening circumstances.
Clinically, adrenaline’s pharmacokinetics and pharmacodynamics demand careful attention. Short half-life, variable absorption rates (depending on route), and potent physiologic impacts necessitate precise dosing regimens and intensive monitoring to optimize benefits while limiting adverse reactions. Its administration can dramatically elevate heart rate, contractility, and bronchodilation, but these life-saving actions carry potential for arrhythmias, hypertension, and metabolic side effects.
An array of factors, from patient comorbidities to co-administered drugs, shape adrenaline’s efficacy and risk profile, underscoring the significance of meticulous clinical judgment. Meanwhile, in scenarios like anaphylaxis, immediate administration of adrenaline alone can be the pivotal edge between life and death. Ongoing research constantly refines adrenaline’s usage, exploring more stable formulations, exploring novel delivery routes, and investigating precision-dosing frameworks that cater to individual genetic or clinical nuances.
In sum, adrenaline rightfully stands as one of modern medicine’s fundamental pharmacologic handiworks—equipped to mobilize vital body systems in emergencies. When prescribed and administered correctly, adrenaline remains indispensable for saving countless lives, upheld by a century’s worth of evolving knowledge that continues to expand and adapt to contemporary medical challenges.
Disclaimer: This article is intended for informational and educational purposes only. It should not replace professional medical advice, diagnosis, or treatment. Always seek guidance from a qualified healthcare professional for specific medical inquiries or decisions regarding adrenaline administration.