Introduction
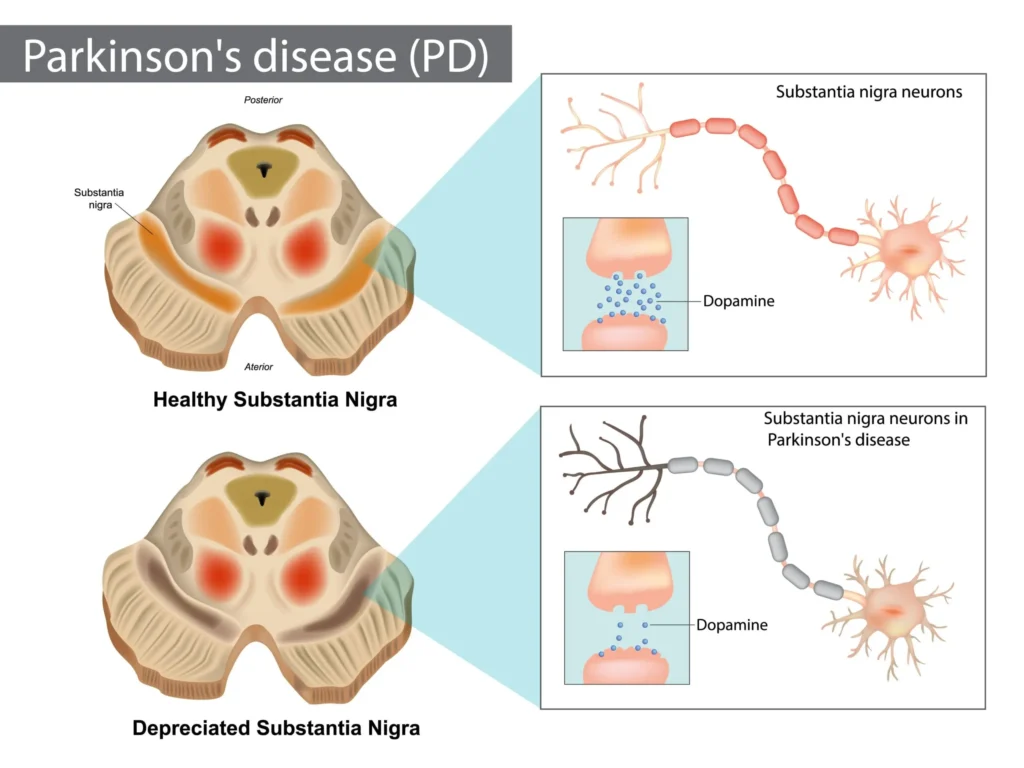
Parkinson’s disease (PD) is a common neurodegenerative disorder characterized predominantly by motor symptoms including bradykinesia, rest tremor, rigidity, and postural instability. These cardinal clinical features trace back to the degeneration of dopaminergic neurons in the substantia nigra pars compacta, leading to reduced dopamine levels within the striatum of the basal ganglia. Along with the hallmark motor findings, patients often experience a variety of non-motor symptoms such as cognitive impairment, psychiatric manifestations, sleep disturbances, autonomic dysfunction, and sensory changes. These non-motor symptoms can significantly affect quality of life and are increasingly recognized as integral to the overall clinical picture (Goodman & Gilman, 2018).
The pharmacotherapy of Parkinson’s disease aims to optimize dopaminergic function or counterbalance the relative overactivity of cholinergic neurons in the striatum. Over time, multiple drug classes have emerged, each addressing unique aspects of the dopaminergic system or targeting relevant non-dopaminergic receptors. The therapeutic landscape includes levodopa (usually paired with carbidopa), dopamine agonists, monoamine oxidase-B (MAO-B) inhibitors, catechol-O-methyltransferase (COMT) inhibitors, anticholinergic agents, amantadine, and newer agents such as adenosine A2A receptor antagonists.
While levodopa/carbidopa remains the most effective treatment for Parkinson’s disease, polypharmacy strategies, which include adjunctive therapies, are often necessary as the disease progresses. Treatments must be carefully individualized due to variations in patient age, comorbidities, severity of disease, and vulnerability to adverse events. In addition, management of motor complications such as “wearing-off,” “on-off” phenomena, and levodopa-induced dyskinesias becomes paramount in advanced stages. This article provides a comprehensive, review of the pharmacotherapy of Parkinson’s disease, spanning the intricacies of pathophysiology, mechanisms of action, clinical usage, adverse effect profiles, and emerging directions as supported by main pharmacology references (Goodman & Gilman, 2018; Katzung, 2020; Rang & Dale, 2019).
Pathophysiology of Parkinson’s Disease
The pathogenesis of Parkinson’s disease is anchored in the degeneration of dopaminergic neurons within the substantia nigra pars compacta, resulting in dopamine depletion across the nigrostriatal pathway. Under normal circumstances, dopamine exerts an inhibitory effect in specific segments of the basal ganglia circuitry, modulating motor output so that movements are executed smoothly and efficiently. As the dopaminergic neurons deteriorate and dopamine concentrations diminish, excitatory pathways (including those driven by acetylcholine) remain relatively unchecked, producing typical Parkinsonian motor signs (Katzung, 2020).
Though dopamine deficiency is core to PD’s clinical presentation, other neurotransmitters—such as acetylcholine, norepinephrine, serotonin, and glutamate—are also dysregulated in the disease. Pathological deposits of Lewy bodies, composed largely of α-synuclein, occur within neurons, contributing to progressive neuronal dysfunction. Genetic studies have illuminated several genes associated with PD (e.g., LRRK2, PINK1), and environmental exposures (e.g., certain pesticides) have been implicated to varying degrees. Nevertheless, most PD cases are considered idiopathic (Rang & Dale, 2019).
In light of these complexities, pharmacotherapy strives to:
• Replenish or supplement dopamine within the basal ganglia.
• Delay the enzymatic breakdown of dopamine.
• Directly stimulate dopamine receptors.
• Balance excessive cholinergic tone.
• Adjust other neurotransmitter systems that modulate motor and non-motor symptoms.By focusing on dopaminergic enhancement, clinicians can partially counteract the pathophysiological deficits and ameliorate the motor aspects of the disease.
Classification of Antiparkinsonian Drugs
Pharmacologic interventions for Parkinson’s disease are broadly classified into the following categories (Katzung, 2020):
- Levodopa (combined with peripheral decarboxylase inhibitor such as Carbidopa)
- Dopamine Agonists (e.g., bromocriptine, pramipexole, ropinirole, rotigotine)
- Monoamine Oxidase Type B (MAO-B) Inhibitors (e.g., selegiline, rasagiline, safinamide)
- Catechol-O-methyltransferase (COMT) Inhibitors (e.g., entacapone, tolcapone, opicapone)
- Anticholinergic Agents (e.g., trihexyphenidyl, benztropine)
- Amantadine and related NMDA receptor modulators
- Adenosine A2A Receptor Antagonists (e.g., istradefylline)
- Adjunctive Approaches: Including potential neuroprotective strategies, gene therapy trials, and combination regimens that target multiple receptor pathways simultaneously.
Although levodopa remains the mainstay for symptomatic relief, many of these additional therapeutic classes help optimize managing the disease’s natural evolution and mitigate levodopa’s eventual motor complications (Rang & Dale, 2019).
Levodopa and Carbidopa: The Gold Standard
Mechanism of Action
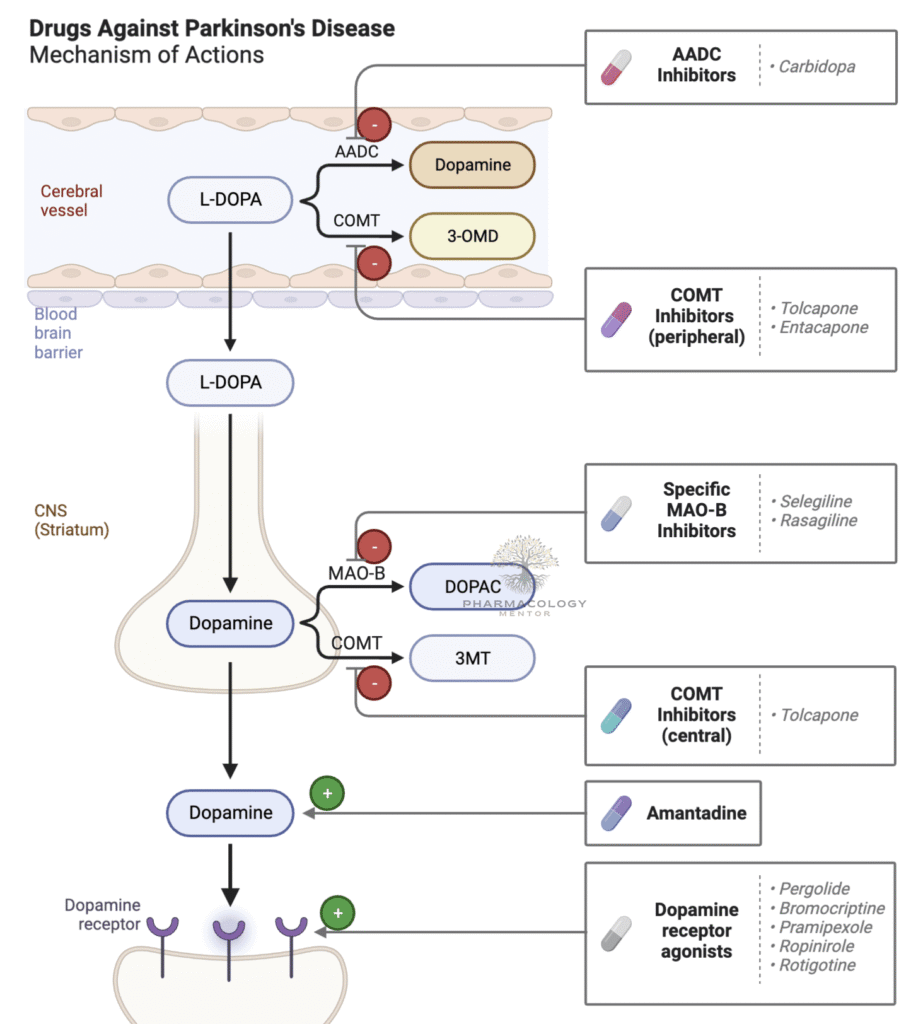
Levodopa (L-DOPA) is a dopamine precursor capable of crossing the blood-brain barrier via L-amino acid transporters. Once in the central nervous system, it is converted to dopamine by the enzyme aromatic L-amino acid decarboxylase (AADC) in dopaminergic neurons. By providing an exogenous source of dopamine, levodopa compensates for the nigrostriatal deficiency that underlies PD’s motor symptoms (Katzung, 2020).
Because significant peripheral conversion of levodopa to dopamine can cause side effects such as nausea, vomiting, cardiac arrhythmias, and hypotension, carbidopa (a peripheral decarboxylase inhibitor) is routinely administered alongside levodopa. Carbidopa blocks peripheral enzyme activity without crossing the blood-brain barrier, increasing the fraction of levodopa that reaches the brain and decreasing peripheral adverse reactions (Goodman & Gilman, 2018).
Pharmacokinetics
• Absorption: Levodopa is absorbed in the small intestine, influenced by gastric motility and competition with dietary amino acids. Patients are often advised to avoid protein-heavy meals around dosing times.
• Metabolism: Outside the CNS, levodopa is metabolized by AADC (blocked by carbidopa) and COMT; within the brain, it is converted to dopamine and then further degraded by MAO-B and COMT.
• Half-Life: The plasma half-life of levodopa, when given with carbidopa, is typically 1–3 hours. This short duration can lead to fluctuating plasma levels and variable symptom control (Rang & Dale, 2019).
Clinical Efficacy
Levodopa/carbidopa remains the most effective pharmacological therapy for Parkinson’s disease. It substantially improves bradykinesia and rigidity, and often tremor, granting patients better overall quality of life. In early disease, the response to levodopa can be dramatic, returning near-normal motor function. However, as PD advances, long-term complications arise even as clinical benefits persist (Katzung, 2020).
Adverse Effects and Complications
• Motor Fluctuations: “Wearing-off” phenomenon, in which each dose of levodopa provides diminishing benefit, leading to “off” times before the next dose. “On-off” phenomena are abrupt, often unpredictable changes in mobility.
• Dyskinesias: Involuntary movements, frequently choreiform, associated with peak dopaminergic activity. They can be debilitating and challenging to manage.
• Peripheral Side Effects: Nausea, vomiting, and orthostatic hypotension (although lessened by carbidopa).
• Neuropsychiatric Issues: Hallucinations, confusion, and even psychosis, especially in older adults or those with preexisting cognitive vulnerabilities (Goodman & Gilman, 2018).
Dose adjustments, use of entended-release formulations, and adjunctive medications (MAO-B or COMT inhibitors) can help mitigate these complications. Nonetheless, levodopa/carbidopa remains the foundational therapy against which other treatments are typically compared.
Dopamine Agonists
Mechanism of Action
Dopamine agonists bypass the need for neuronal storage or conversion of a precursor by directly binding and activating dopamine receptors in the striatum. Several ergot and non-ergot derivatives exist:
- Ergot-based: Bromocriptine (older, less used).
- Non-ergot: Pramipexole, Ropinirole, Rotigotine, Apomorphine.
By stimulating D2-like receptor subtypes, these compounds restore partial dopaminergic function independent of presynaptic neurons (Katzung, 2020).
Pharmacokinetics
Dopamine agonists exhibit varied pharmacokinetic profiles:• Bromocriptine: Undergoes extensive hepatic metabolism; short half-life prompts multiple daily doses.
• Pramipexole: Excreted largely unchanged by the kidneys.
• Ropinirole: Metabolized by CYP1A2 in the liver.
• Rotigotine: Delivered transdermally, offering steady plasma levels and avoiding first-pass metabolism.
• Apomorphine: A rapid-acting agent used frequently as rescue therapy for sudden “off” episodes (Rang & Dale, 2019).
Clinical Use
Dopamine agonists can be used as initial monotherapy in younger patients with mild to moderate symptoms to delay the introduction of levodopa and potentially postpone dyskinesias. In more advanced disease, they often serve as adjuncts to levodopa, reducing “off” periods and possibly enabling lower levodopa doses (Goodman & Gilman, 2018).
Adverse Effects
• Nausea, vomiting, and orthostatic hypotension (due to systemic dopamine receptor effects).
• Excessive Daytime Sleepiness: Patients may experience sudden irresistible urges to sleep, which can be hazardous if driving or operating machinery.
• Impulse Control Disorders: Pathological gambling, hypersexuality, compulsive shopping, and binge eating can occur, sometimes necessitating drug discontinuation.
• Hallucinations, confusion, and psychosis: Especially in the elderly or cognitively impaired (Katzung, 2020).
Monitoring for psychiatric and impulse control side effects is essential, as these can severely disrupt daily functioning.
MAO-B Inhibitors
Mechanism of Action
Monoamine oxidase-B (MAO-B) is an enzyme responsible for oxidative deamination of dopamine in the brain. Selegiline, Rasagiline, and Safinamide selectively bind and inhibit MAO-B at recommended doses, enhancing intracerebral dopamine levels. Higher or nonselective inhibition of MAO can interfere with tyramine metabolism, potentially resulting in hypertensive crises, but selective MAO-B inhibition spares most of these dietary concerns (Goodman & Gilman, 2018).
Representative Agents
• Selegiline (Deprenyl): Partially metabolized to l-amphetamine and l-methamphetamine, which can contribute to minor stimulatory side effects.
• Rasagiline: A more potent and selective MAO-B inhibitor without amphetamine-like metabolites.
• Safinamide: Besides MAO-B inhibition, it modulates glutamate release, possibly broadening its benefits (Rang & Dale, 2019).
Clinical Use
MAO-B inhibitors may be used as monotherapy in early-stage PD to provide mild symptomatic relief and delay the need for levodopa. More commonly, they are employed alongside levodopa/carbidopa in moderate to advanced disease to reduce “off” time and smooth out motor fluctuations (Katzung, 2020).
Adverse Effects
• Generally well tolerated but can exacerbate levodopa-induced dyskinesias or dopaminergic side effects when used together.
• Insomnia, nausea, and headache may occur.
• At higher doses, partial MAO-A inhibition can raise blood pressure or precipitate serotonin syndrome when combined with serotoninergic medications (Goodman & Gilman, 2018).
COMT Inhibitors
Mechanism of Action
Catechol-O-methyltransferase (COMT) metabolizes levodopa into 3-O-methyldopa (3-OMD), diminishing the fraction of levodopa that can cross the blood-brain barrier. By blocking COMT in peripheral tissues (and sometimes centrally), agents such as Entacapone, Tolcapone, and Opicapone preserve levodopa’s bioavailability and prolong its half-life (Rang & Dale, 2019).
Pharmacokinetics
• Entacapone: Primarily acts peripherally; short half-life (~2 hours); given with each dose of levodopa/carbidopa.
• Tolcapone: Longer half-life, crosses the blood-brain barrier to inhibit central and peripheral COMT, but associated with serious hepatotoxicity; requires liver function monitoring.
• Opicapone: Long-acting once-daily formulation, also focuses on peripheral inhibition (Katzung, 2020).
Clinical Use
COMT inhibitors are adjunct therapies for people experiencing wearing-off after each dose of levodopa/carbidopa. By extending levodopa’s duration of action, these agents reduce “off” periods and can lower the total daily requirement of levodopa. Stalevo, a combination of levodopa, carbidopa, and entacapone in a single pill, simplifies treatment regimens (Goodman & Gilman, 2018).
Adverse Effects
• Dyskinesias, hallucinations, and other dopaminergic side effects due to the elevation of levodopa’s effects.
• Diarrhea and urine discoloration (an orange-brown hue) with entacapone.
• Hepatic Injury with tolcapone, necessitating cautious use.
• Potential amplification of orthostatic hypotension (Rang & Dale, 2019).
Anticholinergic Agents
Mechanism of Action
Agents like Trihexyphenidyl (benzhexol) and Benztropine target muscarinic receptors in the striatum. By curtailing the excess cholinergic drive that arises secondary to dopamine deficiency, they relieve symptoms such as tremor and rigidity. These drugs do not significantly help bradykinesia (Katzung, 2020).
Clinical Use
Anticholinergics are historically among the earliest treatments for PD. Today, they are mainly used for younger patients with predominant tremor or as adjunct therapies in those who do not respond adequately to dopaminergic interventions. Given their side-effect profile, older adults or individuals with cognitive challenges often struggle with the resulting anticholinergic burden (Goodman & Gilman, 2018).
Adverse Effects
• Peripheral Anticholinergic Effects: Dry mouth, urinary retention, constipation, blurred vision, and tachycardia.
• Central Anticholinergic Effects: Confusion, drowsiness, memory impairment, disorientation—particularly concerning in elderly patients prone to delirium.
• Exacerbation of Glaucoma: Elevated intraocular pressure (Rang & Dale, 2019).Balancing tremor control against cognitive side effects remains a challenge, limiting the role of anticholinergics in advanced or cognitively vulnerable populations.
Amantadine
Mechanism of Action
Amantadine was initially developed as an antiviral but later discovered to flicker mild to moderate dopaminergic improvements by boosting dopamine release, inhibiting dopamine reuptake, and antagonizing N-methyl-D-aspartate (NMDA) receptors. This NMDA antagonism can reduce glutamatergic overactivity in the basal ganglia, helping to manage levodopa-induced dyskinesias (Katzung, 2020).
Pharmacokinetics
• Well-absorbed orally, reaching peak blood levels in 2–4 hours.
• Eliminated via the kidneys in unchanged form, so dose adjustments are necessary in renal impairment.
• Half-life is roughly 12–15 hours, contributing to a once- or twice-daily dosage schedule (Rang & Dale, 2019).
Clinical Use
Amantadine is employed for mild symptomatic relief of tremor, rigidity, and akinesia in early PD or as an adjunct to reduce dyskinesias in later disease stages. Extended-release formulations specifically target peak-dose dyskinesias related to chronic levodopa use (Goodman & Gilman, 2018).
Adverse Effects
• Livedo Reticularis: Mottled purplish discoloration of the skin, usually on the legs.
• Peripheral Edema, potential orthostatic hypotension.
• Confusion, hallucinations, and insomnia—especially in older patients.
• Possible urinary retention in predisposed individuals (Katzung, 2020).If discontinued abruptly, there is a risk of rebound Parkinsonism or a withdrawal syndrome, making tapering prudent.
Adenosine A2A Receptor Antagonists
Mechanism of Action
Adenosine modulates various neurotransmitters in the brain, including dopamine. Adenosine A2A receptor activation in the striatum has an inhibitory effect on D2 receptor signaling, which can exacerbate Parkinsonian features. Therefore, adenosine A2A receptor antagonists, such as Istradefylline, disinhibit dopaminergic pathways, leading to modest improvements in motor symptoms (Rang & Dale, 2019).
Pharmacokinetics
• Orally administered, with bioavailability that may vary.
• Metabolized chiefly by CYP3A4 in the liver, so strong inducers or inhibitors of CYP3A4 can significantly alter plasma drug levels.
• Long half-life (~80–100 hours), facilitating once-daily dosing (Katzung, 2020).
Clinical Use
Istradefylline received approval in some regions as an adjunct treatment for patients with advanced Parkinson’s disease experiencing “off” episodes despite optimized levodopa therapy. Modest but clinically meaningful decreases in “off” time have been reported, enhancing patient motor function (Goodman & Gilman, 2018).
Adverse Effects
• Dyskinesias can worsen since overall dopaminergic function is boosted.
• Insomnia, hallucinations, nausea, and sometimes anxiety.
• Potential for drug-drug interactions via the CYP3A4 pathway (Rang & Dale, 2019).Although beneficial for some, the net improvement with these agents is often more subtle compared to potent dopaminergic drugs.
Management of Motor Complications
Wearing-Off Phenomenon
“Wearing-off” describes a progressive decline in levodopa’s effect near the end of each dosing interval, driving a resurgence of motor symptoms. It usually indicates disease progression and shortening of levodopa’s effective duration. Strategies include:
• Dose Fractionation: Smaller, more frequent doses of levodopa/carbidopa.
• Add-On Therapies: MAO-B or COMT inhibitors to prolong levodopa’s half-life and reduce off time.
• Controlled-Release (CR) or Extended-Release (ER) formulations.
• Dopamine Agonists: As adjuncts to smooth out fluctuations (Katzung, 2020).
On-Off Phenomena
“On-off” fluctuations are abrupt transitions between states of good mobility (“on”) and severe parkinsonism (“off”), sometimes unrelated to levodopa’s dosing schedule. Apomorphine injections offer rapid rescue from “off” episodes, while advanced treatments such as intrajejunal levodopa infusion or deep brain stimulation (DBS) of the subthalamic nucleus can significantly smooth out these unpredictable shifts (Goodman & Gilman, 2018).
Drug-Induced Dyskinesias
Chronic levodopa therapy leads ~50-80% of patients to exhibit dyskinesias after five years. Key interventions include:
• Lowering Total Levodopa Dose: Possibly offset by adding dopamine agonists or amantadine.
• Amantadine Extended Release: Specifically indicated for levodopa-induced dyskinesia.
• Fractionated Dosing: Uniform distribution of doses may reduce peak concentrations.
• Deep Brain Stimulation: Subthalamic nucleus or globus pallidus internus stimulation to treat refractory dyskinesias (Katzung, 2020).
Non-Motor Symptom Management
While motor symptoms define Parkinson’s disease in the minds of most clinicians, non-motor manifestations frequently degrade quality of life. Treatment approaches include:
Neuropsychiatric Complications
• Depression: Tricyclic antidepressants, SSRIs, or SNRIs. Some older antidepressants have anticholinergic properties that can exacerbate confusion, so care is necessary (Rang & Dale, 2019).
• Psychosis: Pimavanserin (a 5-HT2A inverse agonist) helps manage PD-related psychosis without strong D2 blockade. Low-dose quetiapine or clozapine can be used cautiously if psychosis becomes severe.
• Cognitive Impairment: Rivastigmine, a cholinesterase inhibitor, is approved for mild to moderate dementia in Parkinson’s disease (Katzung, 2020).
Autonomic Dysfunction
• Orthostatic Hypotension: May be aggravated by dopaminergic medications. Midodrine, fludrocortisone, or droxidopa can aid in raising blood pressure.
• Constipation: Often managed by dietary fiber, hydration, or osmotic laxatives.
• Urinary Incontinence or Retention: May need antimuscarinics (in incontinence) or intermittent catheterization (with retention). Balancing such treatments with PD meds is complex.
• Sexual Dysfunction: Can stem from PD itself or from therapies; PDE-5 inhibitors might be beneficial (Goodman & Gilman, 2018).
Sleep Disturbances
• Insomnia: Adjusting nighttime doses of dopaminergic agents, using low-dose sedative-hypnotics if needed.
• REM Sleep Behavior Disorder: Melatonin or clonazepam frequently employed for symptom control.
• Excessive Daytime Sleepiness: Evaluate for obstructive sleep apnea. Switching dopamine agonists or using stimulants (like modafinil) may be considered with caution (Rang & Dale, 2019).By incorporating therapies that emphasize both motor and non-motor facets, clinicians can comprehensively tailor treatment for each patient’s unique presentation.
Special Patient Populations and Considerations
Younger-Onset Parkinson’s Disease
Patients developing PD earlier than 50 years old may be more prone to motor complications when started on levodopa at high doses. A common strategy is using a dopamine agonist initially to delay levodopa introduction. However, younger patients are more susceptible to impulse control disorders. Additional therapies (e.g., MAO-B inhibitors) can supplement early dopamine agonist use (Katzung, 2020).
Elderly or Frail Patients
Older patients with cognitive or autonomic vulnerabilities may not tolerate dopamine agonists well. For them, low-dose levodopa/carbidopa with gradual titration remains fundamental. Anticholinergics are usually avoided due to confusion, memory impairment, and urinary retention risks. Polypharmacy with cardiovascular or other chronic medications also raises the potential for hazardous drug interactions (Goodman & Gilman, 2018).
Comorbid Psychiatric Disorders
Individuals with background psychosis or advanced dementia are especially sensitive to dopaminergic side effects, including hallucinations, delusions, and agitation. It may be necessary to use the minimal effective levodopa dosage and integrate antipsychotic therapy (e.g., pimavanserin, quetiapine, or in resistant cases, clozapine) for symptom control (Rang & Dale, 2019).
Surgical and Advanced Therapies as Adjuncts to Pharmacotherapy
While this discussion focuses primarily on pharmacotherapy, it is important to note that surgical approaches and advanced device-aided therapies can enhance or supplement medication management in refractory Parkinson’s disease:
- Deep Brain Stimulation (DBS): Electrodes implanted into subthalamic nucleus or globus pallidus internus modulate abnormal neurological signals, significantly reducing “off” times and treatment-resistant tremor. DBS can reduce levodopa requirements and alleviate dyskinesias.
- Lesioning Techniques: MRI-guided focused ultrasound or stereotactic radiosurgery for thalamotomy/pallidotomy may benefit unilateral tremor or dyskinesias in highly selected patients.
- Intrajejunal Levodopa Infusion: Provides continuous dopaminergic stimulation, diminishing fluctuations in advanced cases.
- Apomorphine Infusion: Subcutaneous administration to stabilize dopaminergic delivery over extended periods (Katzung, 2020).
These interventions do not replace pharmacotherapy but function as adjunctive resources in patients whose motor complications fail to respond adequately to optimal medical management.
Future Directions in Parkinson’s Disease Pharmacotherapy
Disease Modification and Neuroprotection
An ongoing challenge in Parkinson’s disease research is finding treatments that slow, halt, or reverse dopaminergic neuronal loss. Despite historical enthusiasm for putative neuroprotective drugs such as selegiline, rasagiline, coenzyme Q10, and certain calcium channel blockers, robust clinical trials have yet to confirm definitive disease-modifying benefits (Goodman & Gilman, 2018).
Gene and Cell-Based Therapies
Innovative trials investigate gene therapies that elevate the expression of glial cell line-derived neurotrophic factor (GDNF), bolster aromatic L-amino acid decarboxylase in the striatum, or manipulate α-synuclein misfolding. Stem cell approaches seek to implant dopaminergic progenitors into the striatum. Challenges include immune reactions, graft overgrowth, and ensuring functional integration into host neuronal circuits (Rang & Dale, 2019).
Novel Molecular Targets
• Glutamate Receptor Modulators: Further refining NMDA antagonists without psychotomimetic effects could help manage dyskinesias.
• Iron Chelators: Brain iron accumulation correlates with PD pathology; chelators remain in trial phases.
• Inflammation Inhibitors: Microglial activation in PD sparks interest in targeting neuroinflammatory cascades.
• Synuclein Aggregation Blockers: A leading research area given that Lewy bodies and aggregated α-synuclein underlie disease progression (Katzung, 2020).
Personalized Medicine
Genomic technologies could elucidate differential responses to levodopa or dopamine agonists, guiding the choice of therapy based on a patient’s genetic profile. Biomarkers gleaned from imaging, spinal fluid assays, or blood-based panels may eventually predict disease trajectory, enabling earlier and tailored interventions (Goodman & Gilman, 2018).
Balancing Therapy: Putting It All Together
The pharmacotherapy of Parkinson’s disease demands an iterative, patient-centric approach. Initiation of treatment hinges on symptom severity, patient preferences, and risk tolerance for side effects:
- Early to Mild PD: MAO-B inhibitors (e.g., Rasagiline) or a Dopamine Agonist (e.g., Ropinirole) can be sufficient, delaying the introduction of levodopa. In younger patients battling predominantly tremor, anticholinergics (e.g., Trihexyphenidyl) might help.
- Moderate Symptoms: Levodopa/Carbidopa remains the most potent option, usually started at the lowest effective dose to minimize early motor complications.
- Progression and Motor Complications: Add COMT inhibitors (e.g., Entacapone) or MAO-B inhibitors (e.g., Selegiline) to manage wearing-off. Dopamine agonists or extended-release Amantadine may alleviate dyskinesias.
- Advanced Disease: Comprehensive polypharmacy, possibly with Adenosine A2A Receptor Antagonists (e.g., Istradefylline), rescue Apomorphine for unpredictable “off” episodes, or advanced device therapy (such as DBS or enteral levodopa infusion) (Rang & Dale, 2019).
Attention to non-motor facets is equally crucial. Patients often need separate interventions to manage autonomic dysfunction, mood disorders, cognitive decline, and sleep disturbance. Interdisciplinary support from neurology, psychiatry, geriatrics, physical therapy, speech-language pathology, and occupational therapy is indispensable for holistic care (Katzung, 2020).
Conclusion
Parkinson’s disease remains a progressive, incurable neurodegenerative condition that disproportionately impacts the aging population. Nevertheless, remarkable strides in pharmacotherapy now allow many patients to maintain an acceptable quality of life for years—even decades—post-diagnosis. The cornerstone therapy, levodopa/carbidopa, revolutionized the field by effectively replenishing dopamine in the nigrostriatal pathway. Adjunctive agents—dopamine agonists, MAO-B inhibitors, COMT inhibitors, anticholinergics, amantadine, and adenosine A2A receptor antagonists—aid in refining symptom control, limiting motor complications, and addressing the diversity of patient profiles.
While motor symptoms remain the focus of initial therapy decisions, it is crucial to address the burden of non-motor issues—ranging from psychiatric and autonomic to cognitive dysfunction. Advanced therapies, such as deep brain stimulation and intestinal gel infusions, further support pharmacotherapeutic approaches in refractory cases. A synergy of different medication classes, tailored to disease stage, patient tolerance, and comorbidities, shapes modern treatment paradigms.
Looking forward, the ultimate goal remains to develop disease-modifying strategies that slow or reverse the underlying neurodegenerative process. Until this goal is achieved, clinical practice will continue to rely on carefully orchestrated polypharmacy, vigilant monitoring for adverse events (such as dyskinesias or psychiatric effects), and an individualized approach that integrates both motor and non-motor symptom management. Grounded in findings from essential pharmacology references like “Goodman & Gilman’s The Pharmacological Basis of Therapeutics,” “Katzung’s Basic & Clinical Pharmacology,” and “Rang & Dale’s Pharmacology,” the evolving pharmacotherapy of Parkinson’s disease continues to enhance patient well-being and expand our understanding of this multifaceted neurodegenerative disorder (Goodman & Gilman, 2018; Katzung, 2020; Rang & Dale, 2019).
References
• Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 13th Edition.
• Katzung BG, Basic & Clinical Pharmacology, 14th Edition.
• Rang HP, Dale MM, Rang & Dale’s Pharmacology, 8th Edition.
Parkinsons disease
Medical Disclaimer
The medical information on this post is for general educational purposes only and is provided by Pharmacology Mentor. While we strive to keep content current and accurate, Pharmacology Mentor makes no representations or warranties, express or implied, regarding the completeness, accuracy, reliability, suitability, or availability of the post, the website, or any information, products, services, or related graphics for any purpose. This content is not a substitute for professional medical advice, diagnosis, or treatment; always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition and never disregard or delay seeking professional advice because of something you have read here. Reliance on any information provided is solely at your own risk.

