Introduction
Metronidazole is a widely used antimicrobial agent recognized for its efficacy against anaerobic bacteria and protozoa. Derived from nitroimidazole, metronidazole disrupts the DNA of target microorganisms, halting their replication. It was first introduced in the late 1950s as an antiprotozoal, but manufacturers soon discovered potent antibacterial activity as well. Today, metronidazole remains a mainstay treatment for infections caused by Bacteroides fragilis, other anaerobes, and protozoans like Trichomonas vaginalis and Giardia lamblia. It also plays a critical role in the management of Helicobacter pylori when combined with other antibiotics in triple therapy regimens.
This comprehensive review explores the pharmacology of metronidazole, examining its mechanism of action, antimicrobial spectrum, pharmacokinetics, clinical uses, and safety considerations. Citing key pharmacology textbooks such as Goodman & Gilman’s “The Pharmacological Basis of Therapeutics,” Katzung’s “Basic & Clinical Pharmacology,” and Rang & Dale’s “Pharmacology,” this article highlights how metronidazole has remained an indispensable component of anti-infective therapy for decades.
Chemical Structure and Properties
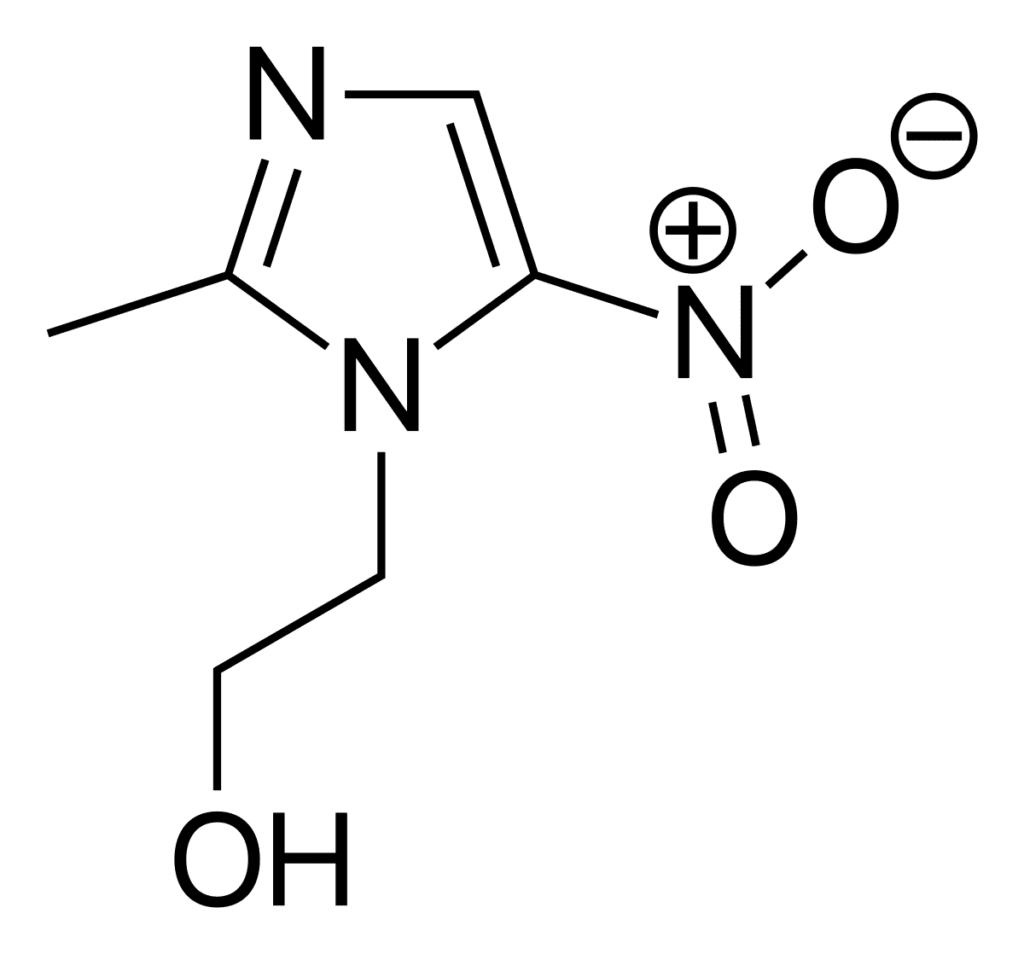
Metronidazole (2-methyl-5-nitroimidazole-1-ethanol) is a nitroimidazole derivative characterized by a nitro group attached to the imidazole ring. This structural feature is critical for its antimicrobial activity. Several physicochemical properties influence its efficacy and formulation:
- Solubility: Metronidazole is moderately water-soluble, enabling intravenous, oral, and topical dosage forms.
- Stability: The compound is generally stable under normal conditions but can degrade in extreme pH environments or upon prolonged exposure to light and heat.
- Bitter Taste: When administered orally, metronidazole has a distinctive bitter taste, which influences formulation strategies (coatings, flavorings) for better palatability.
Mechanism of Action
Selective Uptake and Bioreductive Activation
Metronidazole exerts its antimicrobial effects primarily within anaerobic or microaerophilic microorganisms. Under low-oxygen or strictly anaerobic conditions, microbial enzymes (such as pyruvate:ferredoxin oxidoreductase in protozoa or nitroreductases in bacteria) reduce the nitro group of metronidazole. This reduction generates reactive intermediates, notably nitroso radicals.
Disruption of DNA Synthesis
Once activated, these reactive intermediates bind to microbial DNA, causing DNA strand breaks and loss of the helical structure. This DNA damage effectively halts nucleic acid synthesis, impairing cell replication and leading to cell death. In this way, metronidazole becomes a potent bactericidal and protozoacidal agent. Importantly, because human cells typically operate in oxygen-rich environments, human reductive pathways do not activate metronidazole to the same damaging radicals, conferring relative selectivity.
Oxygen Interference
The presence of oxygen can partially reverse or reduce the drug’s activity because oxygen competes with metronidazole for electrons in the microbial cell. Oxygen re-oxidizes the reactive nitroso radicals back to the parent compound, mitigating DNA damage. This explains why metronidazole demonstrates stronger activity in anaerobic or low-oxygen conditions compared to aerobic environments.
Antimicrobial Spectrum
Activity Against Anaerobes
The hallmark of metronidazole is its excellent coverage against a broad range of anaerobic bacteria, including:
- Bacteroides fragilis and other Bacteroides species.
- Fusobacterium species.
- Clostridium difficile (though resistance can develop).
- Peptostreptococcus species.
These organisms commonly inhabit the gastrointestinal tract and can cause intra-abdominal infections, pelvic infections, and abscesses. Metronidazole frequently pairs with other antibiotics (like fluoroquinolones or cephalosporins) for synergistic or broader-spectrum coverage of mixed infections.
Giardiasis, Trichomoniasis, and Amebiasis
Metronidazole remains the drug of choice for various protozoal infections:
- Trichomonas vaginalis: The CDC recommends a single high dose or a short-course regimen.
- Giardia lamblia: Causes giardiasis, resulting in diarrhea and malabsorption; metronidazole is the first-line therapy.
- Entamoeba histolytica: Responsible for amebiasis, including dysentery and liver abscesses. Metronidazole eliminates trophozoites but typically requires a luminal agent (e.g., paromomycin) for complete clearance of intraluminal cysts.
Helicobacter pylori
In combination triple therapy (commonly metronidazole, a proton pump inhibitor, and clarithromycin or tetracycline/bismuth), metronidazole helps eradicate H. pylori in peptic ulcer disease. Resistance to metronidazole can reduce triple therapy effectiveness, so local resistance patterns may influence regimen selection.
Other Infections
Metronidazole can be used to manage:
- Bacterial Vaginosis caused by anaerobes (particularly Gardnerella vaginalis).
- Rosacea (topical gel formulations).
- Certain orodental infections involving anaerobes.
Pharmacokinetics
Absorption
Metronidazole demonstrates high oral bioavailability (approximately 90%). Oral tablets, capsules, and suspensions quickly achieve therapeutic plasma concentrations. The drug is also given intravenously for moderate-to-severe infections where patients cannot take oral medications.
Distribution
Metronidazole distributes extensively throughout body tissues and fluids, including cerebrospinal fluid (CSF), bile, seminal fluid, saliva, and vaginal secretions. It also reaches high concentrations in abscess cavities, reflecting its lipophilic properties and moderate protein binding (10–20%).
Metabolism
Hepatic metabolism predominates, with metronidazole undergoing oxidation and glucuronidation. Major metabolites (e.g., hydroxymetronidazole) may possess partial antimicrobial activity. The primary enzyme pathways involve CYP2A6 and other isozymes, though they are not as widely studied compared to the metabolism of certain more frequently drug-interacted molecules.
Excretion
Excretion occurs through both the urine (60–80%) and feces (approximately 20%). Trace excretion can tint the urine a dark or reddish-brown color in some patients, a benign phenomenon.
Half-Life
The elimination half-life is roughly 6–8 hours in adults with normal renal and hepatic function. Adjustments may be necessary in significant hepatic impairment, as this can prolong drug half-life and elevate plasma levels. Renal impaired patients generally do not require dose adjustments unless they also have impaired hepatic function.
Clinical Indications
Giardiasis, Trichomoniasis, and Amebic Dysentery
Metronidazole remains the first-line agent for:
- Giardiasis: A short 5–7 day course is typically effective. Single-day high-dose regimens have also been considered.
- Trichomoniasis: A single 2 g dose is recommended by many guidelines. In cases of treatment failure, extended regimens or higher doses can be used.
- Amebiasis: Typically 7–10 days of therapy to eliminate invasive trophozoites, followed by a luminal agent for cyst clearance.
Anaerobic Bacterial Infections
Metronidazole offers potent bactericidal coverage for:
- Intra-abdominal infections (in combination with other broad-spectrum agents).
- Pelvic inflammatory disease or postpartum endometritis.
- Bacterial vaginosis: Oral or intravaginal formulations.
- Skin and soft tissue infections where anaerobes predominate.
C. difficile Infections
Historically, metronidazole was used for mild-to-moderate Clostridium difficile infection (CDI), but current guidelines favor vancomycin or fidaxomicin as first-line oral treatments, especially for severe cases. Metronidazole is now generally reserved for non-severe infections if cost or availability is a concern, or in resource-limited settings.
Helicobacter pylori Eradication
When included in triple or quadruple therapy, metronidazole helps eradicate H. pylori in peptic ulcer disease. Treatment efficacy partially depends on local patterns of metronidazole resistance; therapy failure can occur if resistance rates are high.
Rosacea
Topical metronidazole (creams, gels, lotions) is frequently prescribed for rosacea to reduce inflammation and lesion counts. Its anti-inflammatory properties might be partly responsible for clinical benefit, as the precise mechanism is less about anti-infective action in this context.
Dosage and Administration
Common Adult Oral Doses
- Trichomoniasis: A single 2 g dose or 500 mg twice daily for 7 days.
- Giardiasis: 250 mg–750 mg three times daily for 5–10 days (depending on guidelines and geographic parasite patterns).
- Amebiasis: 750 mg three times daily for 7–10 days.
- Anaerobic infections: 500 mg every 6–8 hours, depending on severity.
- H. pylori triple therapy**: 500 mg two or three times daily (combined with clarithromycin and a proton pump inhibitor), usually for 10–14 days.
(Note: Dosing recommendations can vary among different guidelines, indications, and local susceptibilities. Clinicians should consult up-to-date references.)
Intravenous Regimens
For severe infections or patients who cannot tolerate oral therapy:
- Typical loading dose: 15 mg/kg IV, followed by 7.5 mg/kg IV every 6–8 hours.
- Infusion is generally over 30–60 minutes.
Pediatric Dosing
Pediatric doses vary by weight-based calculations (e.g., 15–30 mg/kg/day in divided doses for Giardiasis or Amebiasis). Clinicians must use specialized references to ensure accurate pediatric dosing based on the infection type and severity.
Side Effects and Adverse Reactions
Gastrointestinal Effects
Nausea, abdominal cramping, metallic taste, and occasional vomiting are the most frequent complaints. Taking the medication with meals can mitigate some GI irritation. The characteristic metallic taste often dissuades patient compliance, so counseling regarding potential flavor changes may help.
Neurological Effects
Metronidazole can provoke neurological reactions in susceptible individuals, particularly if used at higher doses or for longer durations.
- Peripheral Neuropathy typically manifests with numbness or tingling in the extremities.
- Rarely, metronidazole can induce central nervous system toxicity (seizures, encephalopathy), usually reversible upon discontinuation.
Disulfiram-Like Reaction
A well-known effect is the disulfiram-like reaction: Ingestion of alcohol during or immediately following metronidazole therapy may result in flushing, tachycardia, nausea, and vomiting. Although the exact mechanism remains partially debated, patients should abstain from alcohol while on and for at least 24–48 hours after completing metronidazole.
Hypersensitivity
Rashes, pruritus, or urticaria may appear in some individuals. Severe anaphylactic reactions are quite rare. A thorough allergy history prior to treatment is advised.
Hematologic Effects
Leukopenia or neutropenia has been documented, though typically transient. Routine monitoring of complete blood counts is not usually required unless patients need prolonged therapy or have risk factors for hematological abnormalities.
Carcinogenic Potential
Animal studies have indicated that metronidazole possibly has mutagenic or carcinogenic activity in rodents. However, extensive human data do not demonstrate a strong correlation with cancer. Nonetheless, prudent usage (avoiding unnecessary long-term exposure) remains a standard principle.
Drug Interactions
Alcohol
As outlined, metronidazole can lead to a disulfiram-like reaction when combined with ethanol. Patients should avoid any alcoholic beverages or products containing alcohol (even cough syrups) during therapy and for at least 1–2 days post-therapy.
Warfarin and Anticoagulants
Metronidazole may increase plasma levels of warfarin by competing for metabolism pathways (mainly CYP2C9, albeit less extensively). This can intensify anticoagulant effects, elevating the risk of bleeding. Patients often require close monitoring of INR and potential dose adjustments of warfarin during combined administration.
Lithium
Co-administration can lead to increased lithium levels and potential toxicity (manifesting with tremors, confusion, nephrotoxicity). Serum lithium monitoring is recommended if combined therapy is unavoidable.
Phenytoin and Phenobarbital
Enzyme inducers such as phenytoin and phenobarbital can accelerate metronidazole metabolism, diminishing its therapeutic effect. Conversely, metronidazole may alter phenytoin metabolism, and caution around serum phenytoin levels is warranted.
Disulfiram
Concurrent use with disulfiram can produce psychotic reactions and confusion. Generally, at least a 2-week separation is recommended between the last dose of disulfiram and the initiation of metronidazole.
Resistance
Mechanisms of Resistance
Microbial resistance to metronidazole derives from strategies that reduce drug activation or enhance oxygen levels locally. Key resistance mechanisms include:
- Mutations in reductase enzymes, diminishing the formation of reactive intermediates.
- Upregulated oxygen-scavenging processes or decreased drug uptake.
- Horizontal gene transfer of resistance determinants (particularly in certain Bacteroides or Helicobacter strains).
Clinical Impact
While clinically significant resistance among anaerobes remains relatively low, pockets of high-level metronidazole resistance can appear in H. pylori or protozoa in certain geographic regions. This underscores the importance of local susceptibility data and prudent antibiotic stewardship.
Special Populations
Pregnancy and Lactation
Metronidazole falls under pregnancy category B (US FDA classification), indicating no definitive fetal harm in animal studies, yet caution remains. In the first trimester, especially for Trichomonas infections, some guidelines suggest delaying therapy until after 12 weeks gestation if possible. Metronidazole crosses the placenta and is also found in breast milk; transient withholding breastfeeding might be recommended with high single-dose regimens.
Pediatrics
Widely used in pediatric protozoal infections (amebiasis, giardiasis) and certain anaerobic infections. Dosage must be weight-adjusted, as children can metabolize metronidazole differently than adults.
Geriatric Patients
No major differences in safety profile, though hepatic clearance can be reduced in the elderly if comorbid liver dysfunction exists. Vigilant monitoring for neuropathy or GI side effects is prudent.
Immunocompromised Patients
Metronidazole serves critical roles in immunocompromised populations (e.g., HIV, cancer). Potential interactions with immunosuppressants or antiretroviral medications require evaluation on a case-by-case basis. Tolerance is usually acceptable, but peripheral neuropathy risk could be amplified in certain conditions (e.g., advanced HIV).
Safety and Monitoring
Laboratory and Clinical Monitoring
Routine lab monitoring is not mandatory for short courses. However, in extended treatments (over 10–14 days):
- Liver Function Tests (LFTs) can be checked if baseline hepatic compromise is suspected.
- Neurological Assessment for early signs of peripheral neuropathy.
- Complete Blood Count in lengthy treatments, especially with concurrent bone marrow suppression risk.
Overdose Management
Overdose rarely causes severe harm beyond amplified GI and neurological symptoms. Supportive measures (antiemetics, IV fluids) typically suffice, with no specific antidote identified. Seizures or encephalopathy are the most serious concerns but remain uncommon.
Comparative Effectiveness
Versus Clindamycin for Anaerobes
Both metronidazole and clindamycin exhibit strong activity against anaerobes. However, metronidazole generally provides better coverage for Gram-negative anaerobes (Bacteroides, Fusobacterium) and is less associated with C. difficile overgrowth. Clindamycin may excel for some Gram-positive anaerobes and certain allergic scenarios, but has a higher incidence of antibiotic-associated diarrhea.
Versus Tinidazole or Secnidazole
These nitroimidazole derivatives share a similar mechanism to metronidazole but often feature a longer half-life, enabling single-dose regimens for certain protozoal infections. They may be costlier or less available. In practice, metronidazole remains a go-to agent.
Versus Vancomycin for C. difficile
For mild cases of C. difficile infection in resource-limited settings, metronidazole has historically been used. However, oral vancomycin or fidaxomicin outperforms metronidazole in severe cases or recurrences, leading guidelines in many regions to shift away from metronidazole as the initial therapy.
Clinical Pearls and Best Practices
- Take with Food: Minimizes GI upset and unpalatable taste.
- Abstain from Alcohol: Warn patients about potential disulfiram-like reactions, enforcing a strict no-alcohol rule during and 48 hours post-therapy.
- Assess for Neurological Symptoms: Evaluate numbness or tingling if therapy extends beyond 2 weeks or if a patient has underlying neuropathies.
- Tailor Dosing: Adjust the regimen based on indication, local resistance patterns, patient factors. Some short-course or single-dose therapies (especially for trichomoniasis or bacterial vaginosis) are highly effective.
- Combine Appropriately: For polymicrobial infections, pair metronidazole with other agents to cover aerobic Gram-negative rods or resistant Gram-positive organisms.
- Counsel on Taste: Advising on the transient metallic taste helps increase adherence. Helpful strategies include mouth rinses, flavor-masking, or taking the medication with mild food or beverage (non-alcoholic).
- Monitor Warfarin Use: Increased INR or bleeding risk demands closer observation or dosage adjustment of anticoagulants.
Future Directions
Novel Formulations
Research continues exploring controlled-release or better-tasting oral liquid formulations to improve compliance. Enhanced topicals for cutaneous conditions or advanced rectal/vaginal delivery systems might expand current usage.
Resistance Surveillance
As pockets of metronidazole-resistant protozoa or anaerobes grow, consistent susceptibility testing and molecular tracking can guide global health strategies. Development of combination therapies or new molecular scaffolds building on nitroimidazole properties remain under investigation.
Reducing Collateral Damage
Recent interest revolves around modulating gut microbiota disruption during antibiotic therapy. Balancing metronidazole’s potent anaerobic coverage with microbial stewardship fosters an environment that mitigates the likelihood of opportunistic infections like C. difficile.
Conclusion
Metronidazole has proven to be a remarkably versatile agent, spearheading therapy for anaerobic bacterial infections and protozoal diseases for over half a century. Its DNA-disruptive mechanism in hypoxic or anaerobic microbial environments explains its broad reach across Bacteroides species, Clostridium, protozoa, and select microaerophiles such as H. pylori. With favorable oral absorption, IV alternatives, and variable dose regimens, metronidazole stands as a practical yet potent staple in antimicrobial arsenals worldwide.
However, clinicians must carefully respect the drug’s side-effect profile—particularly its gastrointestinal discomfort, risk of neuropathy upon prolonged use, and potential disulfiram-like reaction with alcohol. Evolving guidelines now steer away from metronidazole as first-line prophylaxis or treatment in specific scenarios (e.g., severe C. difficile), highlighting the importance of evidence-based practice and local resistance data. Nevertheless, metronidazole remains indispensable for conditions like trichomoniasis, giardiasis, amebiasis, anaerobic infections, and certain H. pylori regimens, testifying to its enduring value and clinical relevance.
By adhering to best practices—cautious prescribing, monitoring for interactions, educating patients on compliance, and tracking resistance—healthcare professionals can ensure metronidazole continues to provide safe, effective coverage for a spectrum of anaerobic and protozoal infections for years to come.
References
- Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 13th Edition
- Katzung BG, Basic & Clinical Pharmacology, 15th Edition
- Rang HP, Dale MM, Rang & Dale’s Pharmacology, 8th Edition
Medical Disclaimer
The medical information on this post is for general educational purposes only and is provided by Pharmacology Mentor. While we strive to keep content current and accurate, Pharmacology Mentor makes no representations or warranties, express or implied, regarding the completeness, accuracy, reliability, suitability, or availability of the post, the website, or any information, products, services, or related graphics for any purpose. This content is not a substitute for professional medical advice, diagnosis, or treatment; always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition and never disregard or delay seeking professional advice because of something you have read here. Reliance on any information provided is solely at your own risk.

