Introduction
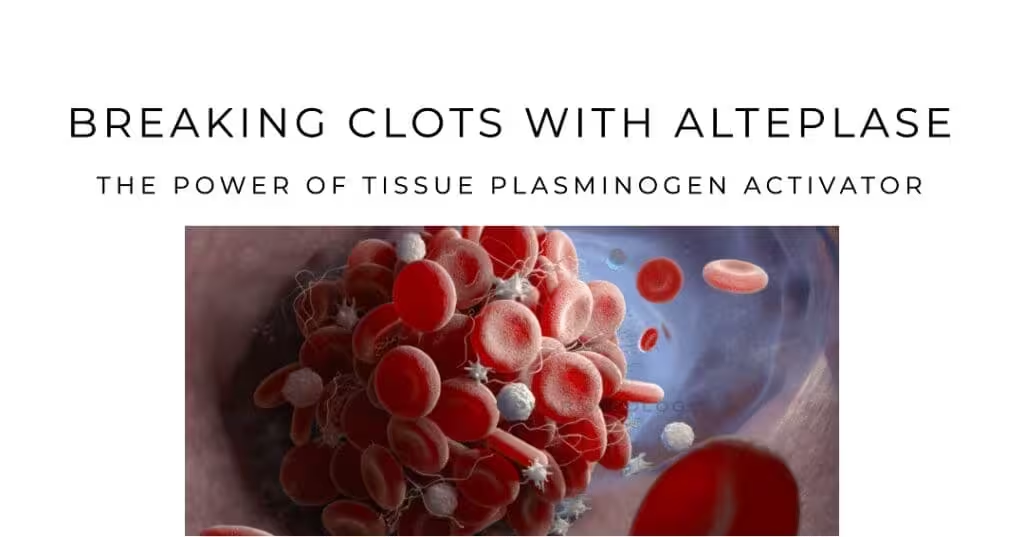
Alteplase (also referred to as recombinant tissue plasminogen activator or rt-PA) is a cornerstone of thrombolytic therapy, widely used to treat acute ischemic stroke, acute myocardial infarction, and other thromboembolic conditions. As a bioengineered form of tissue plasminogen activator (tPA), alteplase exerts its therapeutic effect by converting plasminogen to plasmin, thereby dissolving clots that obstruct blood flow to critical organs. This capacity to promote fibrinolysis has made alteplase invaluable in emergency medicine, offering a potential remedy for otherwise debilitating or life-threatening vascular occlusions.
Despite its clinical significance, the use of alteplase requires careful patient selection, rigorous adherence to guidelines, and instituted protocols to minimize bleeding complications. Clinicians must also be cognizant of key contraindications and monitoring parameters to ensure that the potential benefits of thrombolysis are not overshadowed by hemorrhagic events. This article offers a comprehensive analysis of alteplase, discussing its development, mechanism of action, pharmacokinetic profile, indications, dosage and administration strategies, adverse effects, and future perspectives in thrombosis management.
Historical Background of Fibrinolytic Therapy
The foundation for modern fibrinolytic therapy dates back to observations that certain enzymes could lyse clots. Streptokinase and urokinase, early examples of fibrinolytics, were effective but associated with nonspecific systemic fibrinolysis and variable immunogenic properties. Subsequent research identified tissue plasminogen activator (tPA) as a more selective agent in physiologic fibrinolysis. Genetic engineering techniques later facilitated the production of alteplase (rt-PA), a recombinant form of human tissue plasminogen activator, which matches endogenous tPA’s amino acid sequence but is manufactured in cell culture systems.
Clinical trials in the late 1980s and early 1990s underscored alteplase’s effectiveness in acute myocardial infarction and ischemic stroke. Over time, stringent protocols and more targeted guidelines emerged to ensure safer usage, forming the basis of current thrombolytic therapy practices that prioritize minimizing hemorrhagic risk while maximizing vascular recanalization.
Overview of Hemostasis and Fibrinolysis
Hemostatic Process
The hemostatic process can be broken down into:
- Primary Hemostasis: Platelet plug formation at the site of vessel injury.
- Secondary Hemostasis: Activation of the coagulation cascade, culminating in fibrin generation to stabilize the platelet plug.
While these steps are critical to prevent undue blood loss, excessive clotting may induce pathologic states like stroke or myocardial infarction. Hence, the body also possesses regulatory mechanisms, notably fibrinolysis, to degrade fibrin and restore vascular patency once healing begins.
Fibrinolytic System
Fibrinolysis ultimately hinges on the conversion of plasminogen (an inactive proenzyme) into plasmin, the enzyme responsible for fibrin breakdown. Tissue plasminogen activator (tPA) is crucial to this step, selectively binding fibrin and activating plasminogen near the thrombus surface. By localizing its proteolytic activity to fibrin-bound plasminogen, tPA limits systemic proteolysis and helps confine clot lysis to the area of need. Exogenous administration of alteplase augments this natural mechanism, accelerating clot dissolution in clinical settings of acute thrombosis.
Pharmacology of Alteplase
Structure and Synthesis
Alteplase is a recombinant glycoprotein composed of 527 amino acids, mirroring the endogenous tPA structure. Produced in mammalian cells (Chinese Hamster Ovary cells), it undergoes post-translational modifications (glycosylation) similar to endogenous tPA. This structural similarity underpins its fibrin-specific binding properties and lowers immunogenic risk compared to non-human fibrinolytics.
Mechanism of Action
In the illustration:
- Plasminogen is converted to Plasmin by the action of Alteplase.
- Plasmin then leads to Fibrin Degradation, which helps in dissolving the Blood Clot.
Alteplase binds to fibrin within a thrombus and catalyzes the conversion of plasminogen to plasmin, the main fibrinolytic enzyme. Plasmin degrades fibrin strands, dissolving the clot and enabling recanalization of the occluded vessel. Importantly, fibrin-bound tPA is substantially more active than free tPA in plasma, enhancing specificity for clot-associated plasminogen while reducing systemic proteolysis.In detail:
- Fibrin Binding: Alteplase preferentially attaches to fibrin within the clot.
- Plasminogen Activation: Once bound, it cleaves plasminogen’s Arg560-Val561 bond, releasing active plasmin.
- Fibrin Degradation: Plasmin digestion of fibrin leads to soluble fibrin degradation products, resolving the obstructive clot.
Pharmacokinetic Profile
- Absorption and Distribution: Administered intravenously, alteplase immediately disperses into the systemic circulation. Due to significant hepatic uptake and local consumption at the clot, plasma half-life is short (approximately 5 minutes in the initial phase).
- Metabolism: Circulating alteplase undergoes hepatic catabolism by liver parenchymal cells.
- Half-Life: A biphasic decay is observed, with an initial fast half-life of about 5 minutes and a terminal half-life of roughly 20–30 minutes. Because of the short half-life, continuous infusion is typically required to maintain effective fibrinolysis.
- Excretion: Metabolites are cleared renally, although the majority of drug clearance is via hepatic routes.
Clinical Indications of Alteplase
1. Acute Ischemic Stroke
Alteplase is the primary agent indicated for thrombolysis in acute ischemic stroke within a defined time window (generally within 3–4.5 hours from symptom onset). By dissolving the occluding thrombus, alteplase can significantly improve neurological outcomes. Stringent inclusion and exclusion criteria exist, as hemorrhagic transformation is a severe complication. Key points:
- Time Window: Earlier administration correlates with improved functional recovery.
- Dose: Typically 0.9 mg/kg (max 90 mg), with 10% given as an IV bolus over 1 minute, and the remainder infused over 60 minutes.
2. Acute Myocardial Infarction (ST-elevation MI)
When percutaneous coronary intervention (PCI) is not promptly available, alteplase can be administered intravenously to dissolve coronary artery clots. This approach was historically pivotal, though primary PCI now often supersedes thrombolysis in many centers. Clinical notes include:
- Goal: Achieve reperfusion and preserve myocardial function, ideally if started within 12 hours of symptom onset.
- Regimens: Weight-based dosing schedules, such as an accelerated protocol over 90 minutes.
3. Pulmonary Embolism
In massive or submassive pulmonary embolism with systemic hypotension or right ventricular dysfunction, alteplase may be employed to reduce clot burden and hemodynamic compromise. Careful patient selection is crucial, weighing bleeding risks against life-threatening PE.
4. Catheter Clearance and Peripheral Arterial Occlusions
Alteplase can be used in lower doses to clear occluded central venous catheters or vascular access devices by instilling a small volume into the line (e.g., 1–2 mg dwell). In acute limb ischemia, local intra-arterial infusion may salvage threatened extremities, preceded by thorough imaging and vascular surgery consultation.
5. Other Investigational Uses
On occasion, alteplase sees off-label or investigational use in certain blocked ventriculo-peritoneal shunts or complicated parapneumonic effusions. Large-scale evidence is less robust in these scenarios, so usage is tailored on a case-by-case basis.
Dosage and Administration
Systemic Thrombolysis Protocols
- Acute Ischemic Stroke: IV dose of 0.9 mg/kg (maximum 90 mg). Bolus 10% (1 minute), remainder over 1 hour.
- ST-Elevation Myocardial Infarction (STEMI): Multiple accelerated protocols exist. One typical approach is:
- 15 mg IV bolus
- 0.75 mg/kg (up to 50 mg) over 30 minutes
- 0.50 mg/kg (up to 35 mg) over the next 60 minutes
- Pulmonary Embolism: Common regimen is 100 mg infusion over 2 hours, plus heparin therapy continuity unless contraindicated by bleeding risk.
Catheter-Directed Administration
Lower doses are used for local instillation in occluded catheters. Typically 1–2 mg linger in the catheter for 30 minutes to 2 hours, after which flushing or aspiration attempts are made to restore patency.
Ancillary Measures
- Heparin or Antiplatelets: Concomitant use may vary by indication. For instance, during PE lysis, intravenous heparin is often paused or carefully managed to balance re-thrombosis risk with hemorrhagic concerns.
- Monitoring: Vital signs, neurologic status, ECG, and laboratory values (e.g., fibrinogen, aPTT) are closely monitored.
Special Considerations
- Weight-Based Calculation: For acute ischemic stroke or MI, actual body weight helps determine maximum recommended dosing.
- Avoiding Intramuscular Injections: Minimizing invasive procedures or IM injections during and immediately following alteplase therapy reduces hematoma risks.
- Time Constraints: Efficacy diminishes with delayed presentation, and hemorrhagic complications can increase.
Contraindications and Precautions
Absolute Contraindications
- Active Internal Bleeding: Current or suspected major hemorrhage.
- Intracranial Hemorrhage History: Prior hemorrhagic stroke or structural intracranial lesion (e.g., arteriovenous malformation).
- Severe Uncontrolled Hypertension: Systolic >185 mmHg or diastolic >110 mmHg that cannot be acutely lowered.
- Recent Intracranial or Intraspinal Surgery: Risk of re-bleeding in the surgical site.
- Known Intracranial Neoplasm: Elevated hemorrhage risk.
- Suspected Aortic Dissection: Rapidly lethal if the vessel wall is compromised by thrombolysis.
Relative Contraindications
- Major Surgery or Trauma within the last 2–4 weeks.
- Recent GI Bleeding or active peptic ulcer disease.
- Severe hepatic or renal dysfunction with coagulopathy.
- Prolonged Cardiopulmonary Resuscitation or internal injuries.
Additional Considerations
Detailed checklists and institutional protocols typically guide safe administration. Some factors can shift from absolute to relative contraindications depending on changes in guidelines and emergent contexts.
Adverse Effects of Alteplase
Hemorrhagic Complications
Bleeding is the most significant and feared complication of alteplase. Ranging from minor (e.g., oozing at venipuncture sites) to major (intracranial hemorrhage, retroperitoneal bleeds), hemorrhagic events necessitate strict patient selection and vigilant clinical monitoring. Neurologic decline, headache, or acute changes in level of consciousness post-thrombolysis warrant immediate imaging (usually CT scan) to assess for intracranial bleeding.
Allergic or Anaphylactoid Reactions
Rare in the context of alteplase. More immunogenic fibrinolytics like streptokinase historically had higher rates of allergic responses. Nonetheless, if anaphylaxis occurs, standard supportive measures (epinephrine, antihistamines, corticosteroids) are critical.
Arrhythmias
Restoration of blood flow to ischemic myocardium can precipitate reperfusion arrhythmias, including accelerated idioventricular rhythm or nonsustained ventricular tachycardia. These events are generally transient but must be distinguished from malignant arrhythmias requiring intervention.
Hypotension
Sudden dissolution of large clots or an overall vasodilatory effect from bradykinin release can cause hypotension. Hemodynamic support, intravenous fluids, or vasopressors might be necessary if severe.
Nausea and Vomiting
Though less common, mild gastrointestinal upset or reflex sympathetic changes can trigger emetic episodes.
Monitoring and Post-Therapy Management
Laboratory Tests
- Coagulation Parameters: Fibrinogen levels, aPTT, PT/INR for baseline and post-therapy changes.
- Complete Blood Count: Monitoring hemoglobin/hematocrit.
- Renal and Hepatic Panels: Ensuring organ function is not deteriorating from shock or hemorrhage.
Clinical Assessments
- Neurological Checks: Particularly critical in stroke management, repeated National Institutes of Health Stroke Scale (NIHSS) evaluations help detect hemorrhagic transformations or changes in infarct size.
- Vital Signs and ECG: For arrhythmias, hypotension, or new chest pain.
- Signs of Bleeding: Frequently inspect wound sites, catheters, stools, and bodily fluids for blood.
Adjunctive Antithrombotic Measures
Depending on indication and institutional protocol, heparin infusion or antiplatelet therapy (e.g., aspirin) may resume within a certain timeframe post-alteplase, provided that no major bleeding complications arise.
Comparison with Other Fibrinolytics
Reteplase and Tenecteplase
Reteplase and tenecteplase are genetically engineered variants of tPA, each modified to enhance pharmacokinetics and fibrin specificity:
- Tenecteplase: Extended half-life allows single-bolus administration in STEMI. Shows increased resistance to plasminogen activator inhibitor-1 (PAI-1).
- Reteplase: Less fibrin-specific than alteplase, used often in double bolus for STEMI. Longer half-life than alteplase.
While alteplase remains standard for ischemic stroke, tenecteplase in low doses is being studied for stroke with promising efficacy. For now, alteplase is considered the gold standard in acute stroke care.
Streptokinase
Earlier-generation agent derived from streptococci. Less selective, increased immunogenicity, and risk of hypotension. Rarely used nowadays in developed nations for acute MI, overshadowed by newer fibrin-specific agents like alteplase and tenecteplase.
Future Perspectives in Thrombolytic Therapy
As medicine shifts toward more precise interventions, research continues to refine fibrinolytics. Approaches include:
- Nanoparticle-Conjugated tPA: Potentially enabling site-directed release at the clot, further limiting systemic exposure.
- Extended Window Protocols: Imaging-based selection (e.g., penumbra detection via advanced MRI or CT perfusion) might expand the time window for stroke therapy beyond 3–4.5 hours, in combination with mechanical thrombectomy.
- Adjunct Agents: Substances that might reduce hemorrhagic risk or stabilize vascular endothelium during lysis.
- Genomic Considerations: Pharmacogenomics research has yet to identify robust genetic biomarkers for alteplase response, but future developments could refine candidate selection or dose optimization.
Practical Considerations and Clinical Pearls
- Identify Clear Indication: The best outcomes emerge when alteplase is deployed swiftly in appropriate patients—particularly in acute ischemic stroke or STEMI without feasible immediate PCI.
- Strict Protocol Adherence: Thorough checklists for contraindications and a protocol-driven approach reduce hemorrhagic complications.
- Blood Pressure Control: Meticulous management of elevated BP before and during alteplase infusion is critical; excessively high BP correlates with intracranial hemorrhage risk.
- Post-Alteplase Neurosurveillance: In stroke contexts, frequent neurological checks are mandatory. Any sign of deterioration warrants emergent neuroimaging.
- Cross-Functional Team Management: Collaboration among emergency medicine, neurology, cardiology, and critical care ensures safe administration and real-time complication control.
- Patient Counseling: Informing patients or families about the potential benefits (salvaging tissue/limb) versus bleeding risk fosters informed consent, especially in urgent scenarios.
Conclusion
Alteplase remains a pivotal drug in the management of acute ischemic stroke, selected myocardial infarction cases, pulmonary embolism, and certain local thrombotic occlusions. By replicating the endogenous fibrinolytic mechanism of tPA, it stands out for its fibrin specificity and overall efficacy in dissolving obstructive clots. The short half-life mandates continuous infusion protocols, strict time windows, and well-defined eligibility criteria to balance efficacy with hemorrhagic risk. Moreover, emerging research in pharmacomodulation, drug delivery, and radiologic triage may further harness alteplase’s potential, optimizing reperfusion outcomes while reducing adverse events.
Clinicians who grasp the nuances of alteplase’s mechanism, dosing regimens, contraindications, and monitoring will find it an indispensable component of emergency reperfusion strategies. As guidelines evolve and new evidence accumulates, alteplase remains an enduring testament to the power of recombinant biologics for life-saving therapeutic interventions.
References
- Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 13th Edition
- Katzung BG, Basic & Clinical Pharmacology, 15th Edition
- Rang HP, Dale MM, Rang & Dale’s Pharmacology, 8th Edition
📚 AI Pharma Quiz Generator
🎉 Quiz Results
Medical Disclaimer
The medical information on this post is for general educational purposes only and is provided by Pharmacology Mentor. While we strive to keep content current and accurate, Pharmacology Mentor makes no representations or warranties, express or implied, regarding the completeness, accuracy, reliability, suitability, or availability of the post, the website, or any information, products, services, or related graphics for any purpose. This content is not a substitute for professional medical advice, diagnosis, or treatment; always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition and never disregard or delay seeking professional advice because of something you have read here. Reliance on any information provided is solely at your own risk.