Introduction
Heparin stands as one of the most essential and time-honored anticoagulants utilized in modern medicine. Discovered early in the 20th century, it found rapid acceptance in clinical practice for the prophylaxis and treatment of thromboembolic disorders. By inhibiting various steps in the coagulation cascade, heparin helps prevent the formation and extension of clots, making it invaluable in a wide range of clinical settings—from acute coronary syndromes and venous thromboembolism to major surgeries requiring extracorporeal circulation. Heparin’s unique properties derive from its complex glycosaminoglycan structure, encompassing a diverse range of molecule sizes and activities.
This comprehensive article on the pharmacology of heparin will delve into its historical evolution, classification, biochemical nature, mechanism of action, pharmacokinetics, clinical uses, adverse effects, as well as promising areas for future research.
As an intravenous or subcutaneous agent, unfractionated heparin (UFH) requires careful monitoring and dosage adjustments to ensure therapeutic efficacy while minimizing side effects. Low-molecular-weight heparin (LMWH), produced through controlled depolymerization, offers more predictable pharmacokinetic properties and a lower risk of certain complications, such as heparin-induced thrombocytopenia (HIT). However, both forms carry inherent risks, and clinical vigilance remains paramount. By the end of this article, readers will have a detailed understanding of the intricacies and best practices surrounding heparin use, enabling safe and effective anticoagulant therapy.
History of Heparin
Heparin’s history dates back to 1916, when it was first discovered by Jay McLean, a medical student at Johns Hopkins University, working under the guidance of William Henry Howell. McLean’s initial goal was to isolate a substance that would help prolong the clotting time of blood and thus aid in preventing thrombosis. This groundbreaking find paved the way for further studies, eventually leading to the identification and characterization of heparin as a sulfated polysaccharide with significant anticoagulant activity.
By the early 1930s, scientists and pharmacologists had substantial evidence confirming heparin’s therapeutic potential for anticoagulation in human patients. However, refining the extraction process took considerable effort before heparin was ready for pharmaceutical applications. Early formulations contained impurities that caused adverse reactions, including hemorrhage and allergic responses. Refinements in purification in the 1940s and 1950s made unfractionated heparin (UFH) a mainstay in clinical practice, and soon after, research began into molecular-weight variations, leading to the development of low-molecular-weight heparins (LMWHs).
Today, both UFH and LMWH remain integral in cardiovascular care, surgical settings, and general medicine worldwide. The discovery of heparin also laid the foundation for a deeper understanding of the coagulation cascade, fostering the development of newer anticoagulants such as direct thrombin inhibitors and factor Xa inhibitors. Despite its age and competition from novel oral anticoagulants (NOACs), heparin continues to enjoy widespread usage because it is effective, quickly reversible, and relatively inexpensive compared to many alternatives.
Chemical and Biological Nature of Heparin
Modern-day heparin preparations mainly fall under two broad categories:
- Unfractionated Heparin (UFH): This form of heparin is a heterogeneous mixture of polysaccharide chains of varying lengths. It is the original heparin used in medical practice.
- Low Molecular Weight Heparin (LMWH): Advances in pharmaceutical technology paved the way for fractionating heparin into smaller chains. Examples include enoxaparin, dalteparin, and tinzaparin.
Although the two variants share a common origin—porcine intestinal mucosa or bovine lung in some formulations—UFH and LMWH differ in molecular weight, pharmacokinetics, administration, and monitoring requirements.
Basic Chemistry and Structure
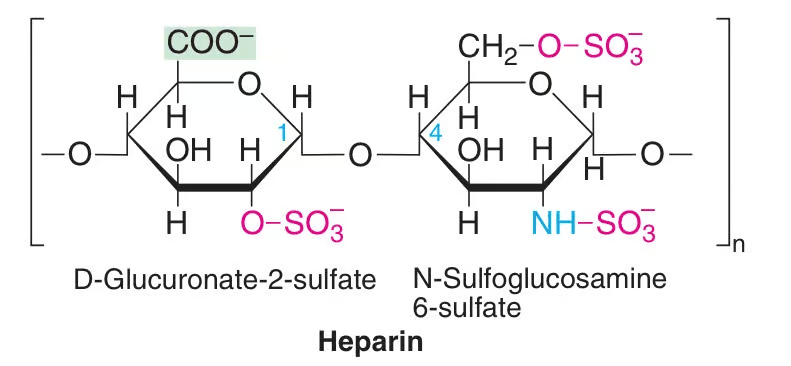
Heparin is essentially a glycosaminoglycan composed of repeating sulfated disaccharide units (uronic acid and glucosamine). The extent of sulfation is instrumental in determining heparin’s binding affinity to proteins like antithrombin (AT) and various coagulation factors. Typically, the disaccharide units are heavily anionic, giving heparin a highly negative charge, which in turn affects its interactions with proteins, cells, and other molecules.
• Unfractionated Heparin (UFH): Polysaccharide chains can range from 3,000 to 30,000 Daltons. Only about one-third of these chains possess the specific pentasaccharide sequence required to bind antithrombin, which is central to heparin’s anticoagulant effect.
• Low Molecular Weight Heparin (LMWH): Created by controlled depolymerization of UFH. The average molecular weight ranges between 3,000 to 8,000 Daltons, making LMWH less heterogeneous and more predictable in its anticoagulant response.
Biological Mechanisms of Action
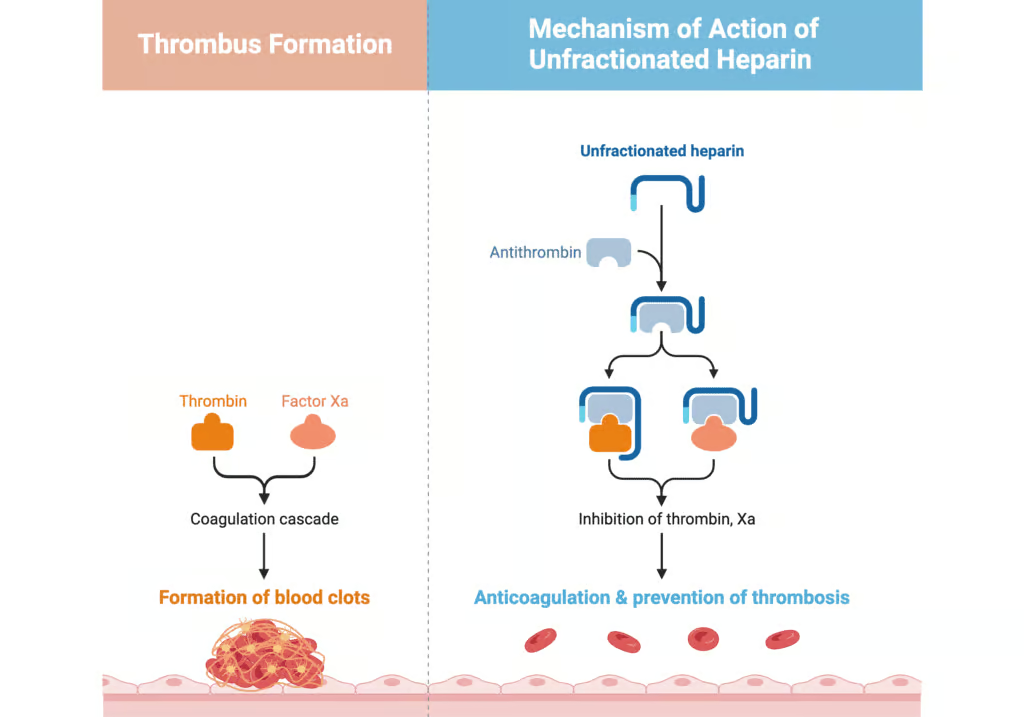
The main anticoagulant property of heparin hinges on its catalytic enhancement of antithrombin’s (AT’s) activity. Let’s break down this critical pathway:
- Antithrombin Binding
– Heparin contains a unique pentasaccharide sequence that tightly binds to and changes the conformation of antithrombin (formerly known as antithrombin III).
– This conformational shift speeds up antithrombin-mediated inhibition of specific clotting factors, especially factor Xa and thrombin (factor IIa). - Inhibition of Factor Xa and Thrombin
– Unfractionated Heparin: Due to its longer average chain length, UFH can bridge thrombin and antithrombin effectively. As a result, UFH potently inactivates both factor Xa and thrombin.
– Low Molecular Weight Heparin: With shorter chains, LMWH predominantly enhances the inhibition of factor Xa by antithrombin but has a more modest effect on thrombin. This factor Xa-selective inhibition contributes to LMWH’s favorable pharmacological profile and lower incidence of certain complications, like heparin-induced thrombocytopenia (HIT). - Overall Anticoagulant Effect
– By inhibiting key clotting factors, heparin reduces the generation of fibrin, thereby halting the formation and extension of blood clots. Unlike other anticoagulants (e.g., warfarin), heparin works immediately upon administration, which is why it’s integral for acute management.
Pharmacokinetics
- Absorption
– Unfractionated Heparin (UFH): When given subcutaneously, UFH absorption can be inconsistent due to the molecule’s large size and polarity. This variation is a key reason why intravenous infusion is often preferred if continuous therapy is needed.
– Low Molecular Weight Heparin (LMWH): LMWH exhibits more reliable subcutaneous absorption (bioavailability around 90%), leading to more predictable plasma concentrations and anticoagulant effects. - Distribution
– Heparin primarily stays within the intravascular compartment because of its large molecular size and strong negative charge, limiting its distribution into tissues.
– Plasma concentrations depend on multiple physiologic variables, including binding to plasma proteins, endothelial cells, and macrophages. - Metabolism and Elimination
– Enzymatic Degradation: Heparin is largely eliminated by the reticuloendothelial system, where it undergoes depolymerization.
– Renal Excretion: Smaller fragments may be excreted by the kidneys. Low molecular weight heparins, being smaller, rely more heavily on renal clearance. Consequently, patients with severe renal impairment require careful monitoring or dose adjustment (especially with LMWH). - Half-Life
– UFH: The half-life ranges approximately 30 to 150 minutes (dose-dependent).
– LMWH: Generally longer half-lives of about 4 to 7 hours, facilitating once or twice daily subcutaneous dosing in many clinical situations. - Special Populations
– Renal Impairment: LMWH can accumulate in patients with renal dysfunction, necessitating dose adjustments or alternative anticoagulants. UFH elimination is less dependent on renal function but still can be affected by reduced clearance.
– Pregnancy: Heparin does not cross the placenta due to its large size and negative charge, making it the anticoagulant of choice for pregnant women requiring anticoagulation.
Pharmacodynamics
- Immediate Onset of Action
One of the hallmark features of intravenous (IV) unfractionated heparin is its rapid onset—the anticoagulant effect starts nearly instantaneously. Subcutaneous administration may take longer to achieve peak effect, typically 20-60 minutes for LMWH and potentially longer for UFH. - Dose-Dependent Efficacy
Heparin’s effect is dose-dependent, meaning higher doses can achieve more potent and sustained anticoagulation. For UFH, continuous IV infusions are common for treating acute thrombotic events, whereas lower doses (often subcutaneous) might be used prophylactically to prevent deep vein thrombosis (DVT). - Monitoring of Coagulation
– Activated Partial Thromboplastin Time (aPTT): The aPTT test is frequently utilized to monitor intravenous UFH therapy. Most protocols aim for an aPTT 1.5–2.5 times the normal control value, indicating therapeutic anticoagulation.
– Anti-Factor Xa Levels: These levels can be used to monitor both UFH and LMWH, although LMWH therapy often does not require routine monitoring in most patients due to its more predictable pharmacokinetic profile. Instead, anti-factor Xa laboratories might be used in special populations (e.g., pregnant women, renally impaired patients, extreme body weight). - Platelet Interaction
Through various mechanisms, heparin can activate platelets or foster the formation of antibodies that trigger heparin-induced thrombocytopenia (HIT). This phenomenon underscores the delicate balance between achieving optimal anticoagulation and reducing the risk of thrombocytopenia or paradoxical thrombosis.
Clinical Indications of Heparin
Heparin finds utility in various acute and chronic clinical settings:
- Treatment of Thromboembolic Disorders
– Deep Vein Thrombosis (DVT): Heparin is a cornerstone for acute management of DVT, often followed by oral anticoagulation for long-term therapy.
– Pulmonary Embolism (PE): By halting further clot propagation, heparin helps stabilize patients with acute PE, reducing mortality. - Acute Coronary Syndromes (ACS)
– In unstable angina or myocardial infarction, heparin is administered to inhibit clot formation within coronary arteries. This approach works alongside antiplatelet agents, improving outcomes. - Cardiovascular Surgery
– Anticoagulation during Bypass: Large heparin doses are administered during cardiopulmonary bypass or extracorporeal membrane oxygenation (ECMO) to keep the circuit free of clots.
– Dosing must be carefully titrated and reversed appropriately after the procedure using protamine sulfate. - Hemodialysis
– Heparin is used to maintain circuit patency in patients undergoing dialysis. UFH is commonly chosen. - Prevention of Thromboembolism
– In hospitalized patients who are bedbound or have limited mobility, prophylactic heparin (typically LMWH) reduces the incidence of hospital-acquired DVT. - Disseminated Intravascular Coagulation (DIC)
– In certain cases, controlled heparin infusion can help manage coagulation abnormalities in DIC, although therapeutic strategies vary depending on the underlying cause and severity.
Dosing and Administration
- Unfractionated Heparin
– Intravenous Infusion: Common for treating acute thrombotic events with a weight-based bolus followed by a continuous infusion. Doses are frequently adjusted based on aPTT or anti-Xa measurements.
– Subcutaneous Injection: Lower doses (5,000 units every 8–12 hours) may be used prophylactically to prevent clots in bedridden patients. - Low Molecular Weight Heparin
– Fixed or Weight-Based Doses: LMWH is administered subcutaneously, often once or twice daily (e.g., enoxaparin 1 mg/kg every 12 hours for DVT treatment). Routine monitoring is not needed except in special populations. - Perioperative Management
– Patients on therapeutic heparin require careful bridging when undergoing surgeries or other invasive procedures. LMWH is frequently utilized due to its easier administration, although timing of last dose and measured anti-Xa levels influence bleeding risk.
Monitoring Parameters
1. Activated Partial Thromboplastin Time (aPTT)
aPTT testing is sensitive to UFH and is traditionally used to guide infusion rates to maintain therapeutic anticoagulation. Levels are drawn at baseline and every 6 hours until stabilized, then at least once daily thereafter.
2. Anti-Xa Activity
Anti-Xa testing provides a direct assessment of factor Xa inhibition by heparin. It can be used for both UFH and LMWH but is especially helpful when aPTT might be confounded—examples include patients on direct thrombin inhibitors, those with lupus anticoagulants, or individuals with hypercoagulable states.
3. Platelet Counts
Regular monitoring of platelet counts is critical due to heparin-induced thrombocytopenia (HIT) risk, a serious immunologic complication. A sudden drop (>50%) from the baseline platelet count or an absolute count of less than 150,000/µL can be a sentinel sign of HIT, initiating an immediate re-evaluation of therapy.
4. Signs of Bleeding
Any unusual bleeding—such as hematomas at injection sites, hematuria, melena, or other hemorrhagic symptoms—should raise immediate concern. Checking hemoglobin and hematocrit levels is a routine part of therapy for detecting occult bleeding.
Adverse Effects and Complications
- Bleeding
Inevitably, as a potent anticoagulant, heparin can cause bleeding complications ranging from mild (hematoma at injection sites) to severe (intracranial hemorrhage). Assessing individual bleeding risk and monitoring relevant lab parameters are key to balancing efficacy with safety. - Heparin-Induced Thrombocytopenia (HIT)
Among the most feared and paradoxical complications is HIT, a prothrombotic disorder triggered by antibodies against platelet factor 4 (PF4)–heparin complexes. This leads to platelet activation and consumption.- Clinical Clues:
– A sudden drop in platelet count by >50% from baseline, typically 5–14 days after starting heparin.
– Risk of new thromboses—arterial or venous—despite being on an anticoagulant.
– Laboratory confirmation involves assays like the serotonin release assay or anti-PF4/heparin ELISA. - Management:
– Immediate discontinuation of all forms of heparin.
– Initiation of a non-heparin anticoagulant (e.g., argatroban, bivalirudin, or fondaparinux) until platelet count recovers. Warfarin should be avoided initially due to the risk of venous limb gangrene and skin necrosis.
- Clinical Clues:
- Osteoporosis
Prolonged heparin use can accelerate bone density loss, contributing to osteoporosis and fractures—especially relevant for pregnant women or others on extended regimens. LMWH may pose a lower risk compared to UFH. - Hypersensitivity Reactions
Allergic reactions, including local skin rashes and systemic anaphylaxis, can occur but are relatively uncommon. - Hyperkalemia
Heparin can interfere with aldosterone production, leading to hyperkalemia in rare instances, especially in those with underlying conditions promoting elevated potassium levels.
Heparin-Induced Thrombocytopenia (HIT)
Given its severity, HIT demands particular attention:
• Incidence
– Occurs in about 1-3% of patients receiving UFH.
– Lower incidence with LMWH, but it remains possible.
• Pathophysiology
– Antibody Formation: The immune system generates IgG antibodies that recognize complexes of PF4 bound to heparin.
– Platelet Activation: These IgG antibodies bind to platelets via Fc receptors, causing platelet activation, microparticle release, and ultimately thrombosis.
• Clinical Consequences
– Thrombocytopenia (platelets often <100,000/µL or a 50% drop from baseline).
– Both venous and arterial thrombotic events can arise (e.g., limb ischemia, stroke, acute myocardial infarction).
• Laboratory Testing
– 4T Score: A clinical scoring system assessing Thrombocytopenia, Timing, Thrombosis, and oTher causes.
– ELISA Test: Detects anti-PF4/heparin antibodies.
– Serotonin Release Assay (SRA): The gold standard confirmatory test, though it’s not widely available in every laboratory.
• Treatment
– Immediate discontinuation of heparin.
– Alternative non-heparin anticoagulants such as argatroban, bivalirudin, danaparoid, or fondaparinux.
– Avoid warfarin until platelet count recovers to at least 150,000/µL to stave off warfarin-induced skin necrosis.
Reversal Agents and Management of Over-Anticoagulation
1. Protamine Sulfate
- This cationic peptide effectively neutralizes heparin’s anticoagulant action by binding to its negatively charged sites.
- Protamine’s effect is almost immediate and lasts about two hours, requiring repeated doses if heparin’s half-life surpasses that time.
- Adverse reactions to protamine include hypotension, bradycardia, and anaphylaxis (particularly in patients with fish allergies or previous vasectomy).
2. Supportive Measures
- In cases of major bleeding, supportive care such as blood product transfusions (packed red blood cells, platelets, or fresh frozen plasma) and local hemostatic interventions may be necessary.
- Reversal of LMWH with protamine is more incomplete than for UFH; the neutralization may be about 60–80% effective.
3. Consideration of Clinical Context
- For mild over-anticoagulation without active bleeding, simply interrupting the heparin infusion and allowing the drug to be cleared might suffice.
- In severe or life-threatening hemorrhages, protamine sulfate is administered carefully, guided by repeated monitoring to fine-tune the neutralization.
Low-Molecular-Weight Heparins vs. Unfractionated Heparin
1. Bioavailability and Dosing
- LMWH: Improved and more predictable bioavailability after subcutaneous injection, allowing for once or twice-daily dosing without routine lab monitoring in most patients.
- UFH: Lower and more variable bioavailability, often requiring IV infusion and frequent aPTT monitoring.
2. Safety Profile
- LMWH: Lower risk of HIT and osteopenia, making it a common choice for outpatient care and prophylaxis.
- UFH: Higher incidence of HIT and osteoporosis with prolonged use, but its short half-life and reversibility can be advantageous in specific settings, like surgeries needing quick anticoagulation adjustments.
3. Clinical Decision Factors
- LMWH is favored in many cases for ease of administration, cost-effectiveness in outpatient management, and fewer complications.
- UFH remains essential for acute inpatient settings where immediate titration and full reversibility are pivotal (e.g., patients requiring urgent surgical procedures or those with severe renal impairment).

Laboratory Monitoring Techniques
- Activated Partial Thromboplastin Time (aPTT)
– Reflects activity of the intrinsic and common coagulation pathways.
– Commonly used to monitor IV UFH therapy.
– Limitations: Variation between laboratories and reagents, lack of linear correlation at very high drug levels. - Anti-Factor Xa Assay
– Measures the inhibitory potential of heparin-activated antithrombin on factor Xa.
– More specific than aPTT for heparin activity, reducing inter-lab variability.
– Advised for: Obesity, renal insufficiency, pregnancy, pediatric populations, and LMWH therapy if monitoring is needed. - Thromboelastography (TEG) or Rotational Thromboelastometry (ROTEM)
– Pinpoints global hemostatic function, tracking the kinetics of clot formation and dissolution.
– Can be useful in complex coagulopathies (e.g., trauma, liver transplantation, or cardiac surgery).
Drug Interactions
- Other Anticoagulants/Antiplatelets
– Combined use of heparin and warfarin, direct oral anticoagulants (DOACs), or antiplatelets (aspirin, clopidogrel) raises bleeding risk. However, bridging therapy with heparin is often standard for initiating warfarin. - Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)
– Concomitant use may increase bleeding risk due to platelet function inhibition. - Oral Antidiabetic Agents
– Heparin does not typically influence blood glucose but watch for potential interactions with other medications that can lead to compromised kidney function in certain individuals. - Thrombolytics
– Heparin is sometimes co-administered during fibrinolytic therapy (e.g., tissue plasminogen activator) in specific situations like acute myocardial infarction or massive pulmonary emboli. This combination boosts the chance of bleeding but also may improve arterial patency.
Transitioning Between Anticoagulants
- Heparin to Warfarin
– Overlap is critical because warfarin takes several days to reach therapeutic levels of vitamin K clotting factor suppression.
– Heparin infusion or LMWH is continued until the international normalized ratio (INR) is in the therapeutic range (often 2–3) for at least 24 hours. - Heparin to Direct Oral Anticoagulants (DOACs)
– Options like rivaroxaban, apixaban, dabigatran, and edoxaban are sometimes used after an initial heparin lead-in for VTE treatment.
– Typically, discontinuation of heparin occurs right before DOAC initiation if labs confirm no significant ongoing heparin effect. - LMWH to UFH (and vice versa)
– Common in perioperative settings or in patients with changing renal function.
– Transition timing depends on the dosing schedule of LMWH (once vs. twice daily) and the aPTT target for UFH.
Special Populations
1. Renal Impairment
- LMWH clearance is more dependent on renal function, therefore dose adjustments or anti-Xa monitoring may be necessary in renal insufficiency.
- UFH remains the safe alternative in severe renal failure because it is mainly cleared by hepatic and reticuloendothelial systems.
2. Pregnancy
- Both UFH and LMWH do not cross the placenta, making them safer than warfarin for preventing or treating venous thromboembolism in pregnant patients. LMWH generally is favored due to lower risk of osteoporosis and better dosing convenience.
3. Obesity
- In obese patients, the subcutaneous absorption of LMWH can be altered. Weight-based dosing is advised. For extremely high body weights, monitoring anti-Xa levels may ensure therapeutic efficacy.
4. Elderly Patients
- The elderly might have age-related renal function declines; dose adjustments or closer monitoring may be needed. They are also at heightened bleeding risk due to other comorbidities and medications.
Osteoporosis and Long-Term Use
Long-term administration of heparin (e.g., in patients with mechanical heart valves who cannot use warfarin or in those who require extended prophylaxis) is linked to bone density loss. The hypothesized mechanisms include decreased osteoblast activity and promotion of osteoclast-mediated bone resorption. Prolonged, high-dose UFH therapy over several months exacerbates this effect, enhancing fracture risks. LMWH, particularly at prophylactic doses, shows a reduced incidence of osteoporosis, although the risk is not zero.Measures to mitigate osteoporosis risk encompass:
- Using the lowest effective heparin dose and switching to oral anticoagulants when feasible.
- Ensuring adequate calcium and vitamin D intake.
- Considering bone density monitoring (DEXA scans) for patients on extended heparin therapy.
Future Perspectives and Ongoing Research
While heparin has been a cornerstone for anticoagulation for nearly a century, advances continue to refine its utility and minimize downsides:
- Development of Longer-Acting Compounds
- Newer LMWH variants and ultra-LMWH are being investigated to further improve bioavailability and reduce side effects.
- Alternative Administration Methods
- Research into oral formulations of heparin has so far been challenging due to poor gastrointestinal absorption and degradation. Nonetheless, novel drug delivery technologies might eventually enable an oral heparin product.
- Biotechnological Derivatives
- Recombinant antithrombin or factor Xa inhibitors could bolster heparin’s actions or provide targeted therapies for specific patient subsets.
- Genetic Insights into HIT
- Identifying at-risk populations for HIT through genetic or proteomic markers may help predict susceptibility and facilitate prophylactic strategies or alternative anticoagulant choices.
- Combinatorial Therapies
- Combining heparin with antiplatelet therapies, fibrinolytics, or novel antithrombotic agents is a continual area of study, aiming to strike an optimal balance between preventing thrombosis and averting bleeding.
Despite emerging anticoagulants, heparin’s unique immediate action, reversibility, and adaptability in diverse clinical scenarios will ensure that it remains an integral tool in healthcare settings.
Practical Tips for Clinicians
- Thorough Risk Assessment: Evaluate each patient’s bleeding risk (e.g., recent surgery, peptic ulcer disease, uncontrolled hypertension) against the risk of thrombosis.
- Use Weight-Based Dosing for LMWH: Avoid fixed dosing in therapeutically anticoagulated patients. Regularly assess body weight changes.
- Check for HIT: Monitor platelet counts regularly, particularly 5 to 14 days after initiating heparin. Watch for sudden drops.
- Renal Function Considerations: Renal insufficiency is a key determinant in LMWH usage. Patients may need dose adjustments or a shift to UFH.
- Educate Patients: Ensuring patients understand the rationale for heparin, signs of bleeding, and the importance of adherence fosters better outcomes.
- Keep Protamine On-Hand: In any acute care setting where heparin is administered, maintain easy access to protamine sulfate and be prepared for rapid infusion if major bleeding arises.
- Stay Alert for New Data: As heparin therapy continuously evolves, remaining updated on guidelines, especially regarding dosing in special populations, ensures safe and effective care.









