Introduction
Epilepsy, a chronic neurological disorder characterized by recurrent seizures, affects millions of individuals worldwide (Katzung, 2020). While the underlying pathophysiology varies, epilepsy generally arises from aberrant, hypersynchronous neuronal discharges in the brain. Such episodes can manifest differently, ranging from brief lapses in awareness to severe convulsions. Pharmacotherapy remains the primary approach to controlling or reducing seizure occurrences, although surgical interventions or neuromodulation may be considered for refractory cases.
Over the past century, numerous antiepileptic drugs (AEDs) have been developed to target diverse seizure types and underlying mechanisms. This article provides a thorough overview of epilepsy pharmacotherapy, examining the classification of seizures, the mechanisms of action and pharmacokinetics of AEDs, standard treatment regimens, adverse effects, and future directions in epilepsy care. Citations are derived from authoritative works such as “Goodman & Gilman’s The Pharmacological Basis of Therapeutics,” “Katzung BG, Basic & Clinical Pharmacology,” and “Rang & Dale’s Pharmacology.”
Classification of Epilepsy and Seizure Types
Epilepsy encompasses a broad spectrum of seizure phenotypes, necessitating a systematic classification. According to the International League Against Epilepsy (ILAE), seizures can be primarily grouped into:
- Focal Onset Seizures (previously partial seizures): Begin in a localized cortical region.
- Focal Aware (motor, sensory, autonomic phenomena without loss of consciousness).
- Focal Impaired Awareness (altered consciousness, automatisms).
- Focal to Bilateral Tonic-Clonic (spreads to both hemispheres).
- Generalized Onset Seizures: Engage both cerebral hemispheres from the start, frequently involving a loss of consciousness.
- Tonic-Clonic (Grand Mal): Characterized by initial tonic rigidity, followed by clonic jerking, and a postictal state.
- Absence (Petit Mal): Brief lapses in consciousness, often with minimal motor manifestations (staring spells).
- Myoclonic: Sudden, brief muscle jerks.
- Atonic: Loss of muscle tone, leading to falls.
- Tonic: Sustained muscle contractions (Katzung, 2020).
Given this variability, choosing the appropriate antiepileptic hinges on the seizure type and underlying etiology (Goodman & Gilman, 2018).
Pathophysiology of Seizure Generation
Seizures originate from an imbalance between excitatory and inhibitory neurotransmission:
- Glutamate: Principal excitatory neurotransmitter. Overactivation (e.g., via NMDA or AMPA receptors) may augment seizure susceptibility.
- Gamma-Aminobutyric Acid (GABA): Main inhibitory neurotransmitter. Diminished GABAergic tone predisposes to hyperexcitability.
- Ion Channel Dysregulation: Sodium (Na⁺), calcium (Ca²⁺), and potassium (K⁺) channels govern neuronal firing thresholds. Mutations or dysfunctions that boost neuronal excitability can trigger seizures (Rang & Dale, 2019).
Most AEDs either reduce excitatory pathways (via glutamate receptor inhibition or Na⁺ channel blockade) or enhance inhibitory circuits (GABA potentiation) (Katzung, 2020).
Mechanisms of Action of Antiepileptic Drugs
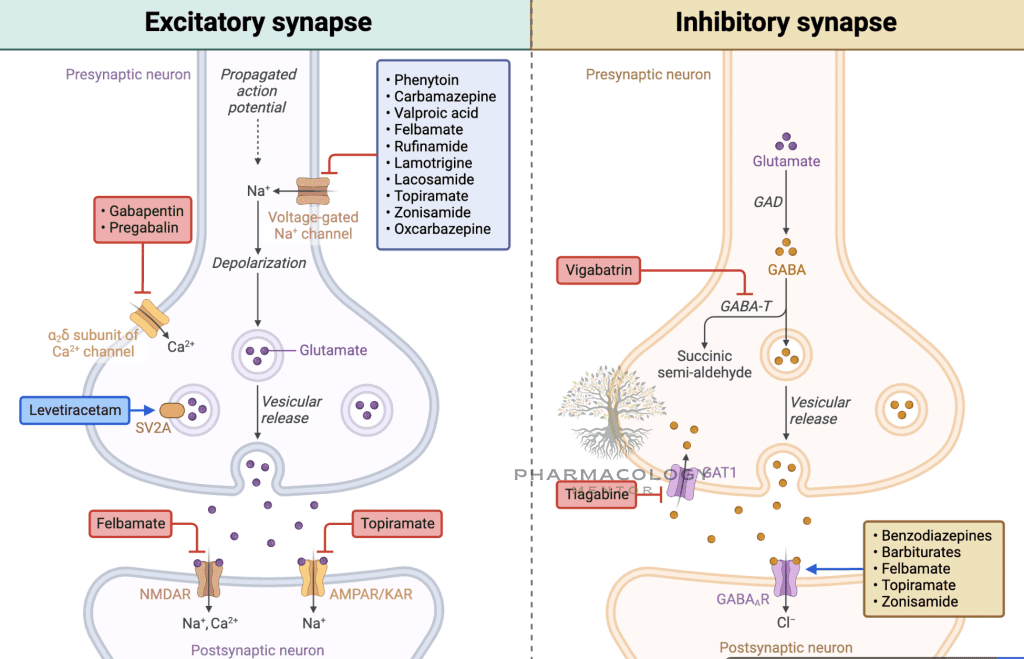
Sodium Channel Blockers
Phenytoin, carbamazepine, lamotrigine, and others stabilize the inactivated state of voltage-gated sodium channels, limiting high-frequency repetitive firing in hyperexcitable neurons. By selectively inhibiting rapidly firing neurons, these drugs curb the initiation and spread of seizure discharges (Goodman & Gilman, 2018).
- Phenytoin: Non-linear kinetics, requires careful monitoring of plasma levels. Adverse effects include gingival hyperplasia, hirsutism, ataxia.
- Carbamazepine: Also used for trigeminal neuralgia and bipolar disorder. Can induce hepatic enzymes, prompting drug interactions.
- Lamotrigine: Broad-spectrum agent useful in focal and generalized seizure types, though it can cause skin rashes (occasionally life-threatening, e.g., Stevens-Johnson syndrome).
Calcium Channel Modulators
- Ethosuximide: T-type Ca²⁺ channel blockade in thalamic neurons, effective in absence seizures.
- Gabapentin, Pregabalin: Structural analogs of GABA that inhibit presynaptic Ca²⁺ channels (α2δ subunit), reducing excitatory neurotransmitter release (Katzung, 2020).
GABA Potentiation
Antiepileptic agents enhance GABAergic inhibition, either by boosting GABA receptor activity or prolonging GABA availability in synapses:
- Benzodiazepines (e.g., diazepam, lorazepam, clonazepam): Enhance frequency of GABA-A receptor Cl⁻ channel opening. Commonly employed in acute seizure cessation (status epilepticus).
- Barbiturates (e.g., phenobarbital): Increase duration of GABA-A receptor chloride channel opening.
- Vigabatrin: Irreversibly inhibits GABA transaminase, elevating GABA levels.
- Tiagabine: Inhibits GABA reuptake transporter (GAT-1), prolonging GABA’s synaptic action (Goodman & Gilman, 2018).
Mixed or Multifactorial Mechanisms
- Valproate (Valproic Acid): Blocks Na⁺ channels, suppresses T-type Ca²⁺ currents, and augments GABA levels (via GABA transaminase inhibition). Broad-spectrum, effective for generalized and focal seizures.
- Topiramate: Blocks Na⁺ channels, enhances GABA, antagonizes AMPA/kainate glutamate receptors. Also used for migraine prophylaxis.
- Levetiracetam: Binds the synaptic vesicle protein SV2A, modulating neurotransmitter release. Effective in multiple seizure types (Rang & Dale, 2019).
- Felbamate: NMDA receptor antagonist with GABA-A receptor potentiation but limited use due to the risk of aplastic anemia, severe hepatotoxicity.
- Perampanel: Selective, noncompetitive AMPA receptor antagonist curbing excitatory glutamate transmission (Katzung, 2020).
Pharmacokinetics and Administration of AEDs
Absorption and Bioavailability
Most AEDs exhibit good oral bioavailability, often exceeding 70–90%. Dosing regimens aim for steady plasma concentrations conducive to seizure control. Some, like carbamazepine, have limited solubility and rely on slow hepatic metabolism for consistent drug levels (Goodman & Gilman, 2018).
Protein Binding
Agents such as phenytoin, valproate, and tiagabine display high plasma protein binding. Co-administration with other highly protein-bound drugs can alter free drug levels, risking toxicity or subtherapeutic effects. The fraction of free (unbound) drug is typically the active moiety (Rang & Dale, 2019).
Metabolism
- CYP450 Inducers: Phenytoin, carbamazepine, phenobarbital can accelerate the metabolism of co-administered drugs, including other AEDs and oral contraceptives.
- CYP450 Inhibitors: Valproate can inhibit the metabolism of certain drugs, raising the potential for toxicity.
- Autoinduction: Carbamazepine fosters its own metabolism, necessitating progressive dose adjustments (Katzung, 2020).
Half-Life and Steady State
Many AEDs have intermediate half-lives (~8–40 hours). Reaching steady-state typically necessitates about 5 half-lives. Agents like phenobarbital have lengthy half-lives (up to 90–100 hours), while others (including levetiracetam) may be shorter (Goodman & Gilman, 2018).
Special Formulations
- Controlled-Release formulations (e.g., carbamazepine ER) reduce peak-trough variations, potentially minimizing side effects.
- IV forms of phenytoin (fosphenytoin) or valproate facilitate acute status epilepticus management (Katzung, 2020).
Choosing an Antiepileptic Drug
Focal Seizures
First-line therapies often include carbamazepine, lamotrigine, levetiracetam, phenytoin, or valproate. Oxcarbazepine or lacosamide represent newer options with potentially fewer interactions. Gabapentin and pregabalin are typically adjunctive in focal epilepsies (Rang & Dale, 2019).
Generalized Tonic-Clonic Seizures
Valproate, lamotrigine, levetiracetam, and topiramate frequently anchor initial therapy. Phenytoin or phenobarbital may also be employed, albeit more historical. Comorbidities and side effect profiles drive final selection (Goodman & Gilman, 2018).
Absence Seizures
- Ethosuximide remains the agent of choice for uncomplicated absence in children.
- Valproate suits patients with co-occurring generalized tonic-clonic or myoclonic seizures (Katzung, 2020).
Myoclonic Seizures
Valproate, levetiracetam, and benzodiazepines (clonazepam) effectively control myoclonic jerks. Topiramate may also be recommended as monotherapy or adjunct (Rang & Dale, 2019).
Atonic or Tonic Seizures
Often challenging to manage, these seizure types respond to valproate, lamotrigine, or topiramate. Adjunctive therapy with rufinamide or the benzodiazepine clobazam is sometimes beneficial for Lennox-Gastaut syndrome (Goodman & Gilman, 2018).
Status Epilepticus
A neurological emergency requiring:
- Benzodiazepines (IV lorazepam or diazepam) for rapid seizure cessation.
- Loading with IV Fosphenytoin, Valproate, or Levetiracetam if seizures persist.
- Refractory Cases: Potential infusion of midazolam, pentobarbital, or propofol under EEG monitoring (Katzung, 2020).
Adverse Effects of Antiepileptic Drugs
CNS Effects
- Sedation, dizziness, ataxia, nystagmus are common with many AEDs, especially on initiation or dose escalation.
- Cognitive Impairment: Varies by drug. Topiramate can produce word-finding difficulties; phenobarbital is known for sedation and slowed cognition (Rang & Dale, 2019).
Dermatologic Reactions
Rashes ranging from mild to severe (e.g., Stevens-Johnson syndrome, toxic epidermal necrolysis) are a serious concern with phenytoin, carbamazepine, lamotrigine, phenobarbital. Titration strategies with slow dose escalation reduce these risks (Goodman & Gilman, 2018).
Hematological Toxicities
- Carbamazepine can cause leukopenia, aplastic anemia (rare).
- Felbamate is linked to aplastic anemia, restricting its use to severe, refractory cases.
Hepatotoxicity
Valproate, felbamate, and lamotrigine (rarely) can cause hepatic injury. Periodic liver function monitoring is recommended, especially in children or early in therapy (Katzung, 2020).
Metabolic and Endocrine Effects
- Weight Gain: Valproate, gabapentin, pregabalin, vigabatrin can induce weight gain.
- Weight Loss: Topiramate, zonisamide can reduce appetite.
- Bone Health: Phenytoin or carbamazepine might accelerate vitamin D metabolism, increasing the risk of osteoporosis.
- Teratogenicity: Particularly prominent with valproate (e.g., neural tube defects). Topiramate also confers elevated risk of cleft palate. Women of childbearing potential require counseling on contraception and folic acid supplementation (Goodman & Gilman, 2018).
Behavior and Mood Changes
Some AEDs (e.g., levetiracetam, perampanel) have been implicated in irritability or mood changes. Benzodiazepines can cause sedation and dependence. Monitoring mental status is crucial (Rang & Dale, 2019).
Drug Interactions and Special Considerations
Inducers and Inhibitors
- Enzyme Inducers: Carbamazepine, phenytoin, phenobarbital, primidone expedite metabolism of numerous drugs (oral contraceptives, warfarin, etc.).
- Enzyme Inhibitors: Valproate can heighten plasma levels of lamotrigine or phenobarbital.
Oral Contraceptives
Estrogen-containing contraceptives can reduce lamotrigine levels, while enzyme-inducing AEDs can reduce contraceptive efficacy. Lamotrigine or levetiracetam often exert fewer hormonal interactions (Katzung, 2020).
Geriatric Populations
Heightened sensitivity to CNS side effects, sedation, and fall risk require cautious titration. Lower protein binding and comorbidities (renal impairment) may alter drug clearance. Favoring newer AEDs with fewer interactions—like levetiracetam or lamotrigine—can be beneficial (Rang & Dale, 2019).
Pediatric Populations
Metabolic rates differ in children, often necessitating higher weight-based adult doses. Avoid valproate in very young children at risk for hepatic toxicity. For absence seizures, ethosuximide is first-line, but add valproate if GTC coexists (Goodman & Gilman, 2018).
Pregnancy and Epilepsy
Pregnant women with epilepsy require close management. Key points include (Katzung, 2020):
- Teratogenic Risks: Valproate poses the highest known risk for neural tube defects and cognitive impairment. Carbamazepine and phenytoin also hold certain risks, though comparatively less. Lamotrigine and levetiracetam are often regarded as relatively safer.
- Folic Acid Supplementation: Minimizes neural tube defects; recommended for all women of childbearing potential on AEDs.
- Drug Level Fluctuations: Physiological changes in pregnancy can lower lamotrigine or levetiracetam levels. Monitoring and dose adjustments are essential.
- Vitamin K: Some practitioners recommend vitamin K supplementation in the third trimester if the mother is on enzyme-inducing AEDs to reduce neonatal hemorrhage (Rang & Dale, 2019).
Strategies for Monotherapy vs. Polytherapy
Monotherapy is ideal for better compliance, minimized side effects, and reduced drug-drug interactions. If a single AED fails to provide adequate seizure control (after verifying compliance and dose adequacy), switching to another agent is considered (Goodman & Gilman, 2018).
Polytherapy may be necessary for complex or refractory epilepsies (e.g., Lennox-Gastaut). Rational combinations exploit different mechanisms of action without overlapping toxicities—examples include pairing valproate (GABA enhancement, Na⁺ blockade) with levetiracetam (SV2A binding) or topiramate (multimodal) (Katzung, 2020).
Drug Withdrawal and Discontinuation
For patients who have been seizure-free for two or more years, tapering AEDs can be considered. However:
- Seizure Type: Primary generalized or childhood absence seizures have better remission rates.
- EEG Normalization: A normal EEG can suggest successful withdrawal.
- Gradual Tapering: Over weeks to months to reduce relapse risk (Rang & Dale, 2019).
Relapses occur in ~20–40% of cases; the decision must weigh risks of recurrence against drug side effects, with close follow-up.
Management of Refractory Epilepsy
An estimated 20–30% of patients remain drug-resistant despite optimal AED regimens (Katzung, 2020). Additional interventions include:
- Surgical Resection: Suitable for focal-onset, well-localized epileptogenic zones (temporal lobectomy).
- Ketogenic Diet: High fat, low carbohydrate regimen that modifies brain metabolism. Effective in pediatric refractory epilepsy.
- Neurostimulation: Vagus nerve stimulation or implantable responsive neurostimulation can moderate seizure frequencies.
- Novel AEDs: Agents like cannabidiol have gained traction particularly in Dravet syndrome or Lennox-Gastaut syndrome (Goodman & Gilman, 2018).
Monitoring and Long-Term Care
Therapeutic Drug Monitoring
- Phenytoin, valproate, carbamazepine, phenobarbital levels are often checked, ensuring therapeutic ranges and avoiding toxicity. For newer AEDs like levetiracetam or topiramate, routine level measurement is less common unless concerns arise (Rang & Dale, 2019).
Side Effect Surveillance
Regular follow-up visits assess sedation, cognition, mood changes, dermatologic status, weight fluctuations, and organ function labs (hepatic, renal) especially for older-generation AEDs (Katzung, 2020).
Psychosocial Support
Epilepsy’s impact extends to driving privileges, employment prospects, mental health, and social dynamics. A collaborative care model integrates medication adherence, counseling, and lifestyle modifications. Medication compliance is essential to maintain steady anticonvulsant effects (Goodman & Gilman, 2018).
Future Directions and Research
- Novel Targets: Agents focusing on KV7 potassium channels, selective NMDA receptor subunits, or neuroinflammation pathways are under investigation.
- Gene Therapy: For monogenic epilepsies (e.g., Dravet syndrome caused by SCN1A mutations), gene therapies or antisense oligonucleotides may hold promise.
- Personalized Medicine: Genotype-driven approaches enabling tailored AED selection—particularly regarding enzyme polymorphisms (CYP2C9, CYP2C19) or ion channel mutations.
- Biomarkers: EEG patterns, imaging techniques, or proteomic signatures could refine seizure prediction and drug response monitoring (Rang & Dale, 2019).
Conclusion
Epilepsy pharmacotherapy stands as an evolving field dedicated to controlling a complex, multifactorial neurological disorder. By leveraging diverse mechanisms—sodium or calcium channel blockade, GABA potentiation, glutamate inhibition—today’s antiepileptic drugs can accommodate most seizure types. Nevertheless, careful consideration of efficacy, side effects, drug interactions, patient age, comorbidities, and reproductive status guides optimal therapy.
Advances in new molecular targets and personalized approaches offer hope for improved seizure control and reduced toxicity in refractory populations. Ultimately, successful epilepsy management demands not just the right drug but a comprehensive, patient-centered strategy integrating lifelong monitoring, psychosocial support, and collaboration with specialized epilepsy care teams (Katzung, 2020; Goodman & Gilman, 2018; Rang & Dale, 2019).
References
- Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 13th Edition.
- Katzung BG, Basic & Clinical Pharmacology, 14th Edition.
- Rang HP, Dale MM, Rang & Dale’s Pharmacology, 8th Edition.
📚 AI Pharma Quiz Generator
🎉 Quiz Results
Medical Disclaimer
The medical information on this post is for general educational purposes only and is provided by Pharmacology Mentor. While we strive to keep content current and accurate, Pharmacology Mentor makes no representations or warranties, express or implied, regarding the completeness, accuracy, reliability, suitability, or availability of the post, the website, or any information, products, services, or related graphics for any purpose. This content is not a substitute for professional medical advice, diagnosis, or treatment; always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition and never disregard or delay seeking professional advice because of something you have read here. Reliance on any information provided is solely at your own risk.