Linezolid is a synthetic oxazolidinone antibiotic indicated for serious Gram-positive bacterial infections, including those caused by methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus faecium (VRE), and penicillin-resistant Streptococcus pneumoniae. It is unique for its mechanism of action, broad tissue penetration, high oral bioavailability, and activity against multidrug-resistant pathogens.
Chemical Structure and Properties
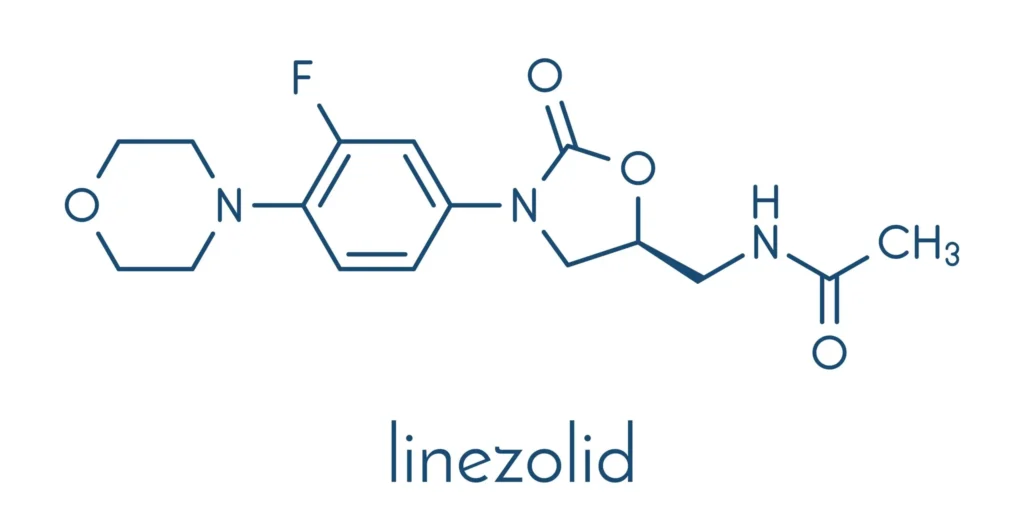
Chemically, Linezolid is known as N-[[3-(3-Fluoro-4-morpholin-4-ylphenyl)-2-oxo-5-oxazolidinyl]methyl]acetamide. As a synthetic antibiotic, its structure is distinct from other antibacterial classes. This uniquene
Mechanism of Action
Linezolid acts by binding to the 23S ribosomal RNA of the 50S subunit, preventing the formation of the functional 70S initiation complex during bacterial translation initiation. This inhibits the first step of protein synthesis, distinct from the mechanisms of other protein synthesis inhibitors such as macrolides and lincosamides, which affect elongation or translocation steps. Linezolid is bacteriostatic against staphylococci and enterococci but bactericidal against most streptococci.
Antimicrobial Spectrum
Linezolid covers aerobic Gram-positive bacteria—including MRSA, VRE, Streptococcus pneumoniae, Streptococcus pyogenes, and Streptococcus agalactiae—but is ineffective against Gram-negative organisms and should be combined with other agents if Gram-negative coverage is required.
Indications
FDA-approved indications include:
- Nosocomial pneumonia (MRSA, Streptococcus pneumoniae)
- Community-acquired pneumonia
- Complicated and uncomplicated skin and skin structure infections (including diabetic foot infections without osteomyelitis)
- Vancomycin-resistant Enterococcus faecium infections (including bacteremia)
Linezolid is also used off-label for multidrug-resistant tuberculosis (MDR-TB) in select regimens.
Pharmacokinetics
Linezolid has very high (≥90%) oral bioavailability; oral and IV dosing are interchangeable on a 1:1 basis. Peak plasma concentration occurs 1–2 hours after ingestion. The drug distributes widely—including into lungs, tissues, CSF, and fluids—facilitated by moderate protein binding (~30%). It is metabolized by oxidation of the morpholine ring and excreted via the urine; around 30% is excreted unchanged, and the half-life is 5–7 hours. No dose adjustment is generally required for mild-to-moderate renal or hepatic impairment.
Dosing
- Adults: 600 mg IV or PO every 12 hours for 10–14 days (up to 28 days for VRE infections)
- Pediatric dosing is weight-based and similar in frequency
- Direct IV-to-PO switch is allowable thanks to high bioavailability
Adverse Effects
- Hematologic toxicity (myelosuppression, anemia, thrombocytopenia): Monitor CBC weekly, especially after >2 weeks of therapy.
- Neuropathy, lactic acidosis: With prolonged (>28 days) use.
- Serotonin syndrome: Due to reversible nonselective MAO inhibition; avoid use with serotonergic and adrenergic drugs.
- Gastrointestinal upset: Nausea, vomiting, diarrhea.
- Rash and allergic reactions: Uncommon but reported.
Drug interactions
Linezolid is a weak, reversible, nonselective monoamine oxidase inhibitor (MAOI). It can interact dangerously with serotonergic agents (SSRIs, SNRIs, TCAs, tramadol, etc.) and sympathomimetics, leading to serotonin syndrome or hypertensive crisis. Tyramine-rich foods may cause hypertension.
Contraindications
- Known hypersensitivity or allergy to linezolid or other oxazolidinones.
- Coadministration with serotonergic or adrenergic drugs unless dose reduction and close monitoring are possible.
Resistance
Resistance typically arises via mutations in domain V of the 23S rRNA or acquisition of methyltransferase enzymes encoded by cfr genes. Cross-resistance with other protein synthesis inhibitors is rare.
Special Populations
- Pregnancy: Crosses placenta; use only if potential benefit justifies risk.
- Lactation: Insufficient data; caution advised.
- Renal/Hepatic impairment: Generally, no dose adjustment required, but monitor closely in severe dysfunction.
Clinical Pearls
- Seamless IV-to-PO switch improves cost-effectiveness and discharge planning.
- Drug monitoring may be considered for prolonged or high-risk use due to myelosuppression risk.
- Lacks Gram-negative activity; always check what pathogens need coverage.
- Especially valuable for multidrug-resistant Gram-positive infections when alternatives (vancomycin, daptomycin) are contraindicated, poorly tolerated, or ineffective.
References
- Azzouz A, Murphy PB, Stevenson B, et al. Linezolid. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Feb 29.
- Brunton LL, Hilal-Dandan R, Knollmann BC, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 14th ed. New York: McGraw-Hill; 2022.
- Johns Hopkins ABX Guide. Linezolid. Updated 2024.
- DrugBank. Linezolid: Uses, Interactions, Mechanism of Action. Updated 2022 Oct 30.
- Clinical Policy: Linezolid (Zyvox). Centene Corporation; Revised 2021 Jun 30.
- Nugraha RV. Linezolid as a Treatment for Multidrug-resistant Tuberculosis. Kneopen; 2024.
- Springer Nature. Serotonin syndrome by drug interactions with linezolid: clues from pharmacovigilance-pharmacokinetic/pharmacodynamic analysis. 2020 Sep 7.
- Yang W, et al. Resistance to linezolid in Staphylococcus aureus by mutations in the 23S rRNA. Nature; 2024 Oct 17.
Medical Disclaimer
The medical information on this post is for general educational purposes only and is provided by Pharmacology Mentor. While we strive to keep content current and accurate, Pharmacology Mentor makes no representations or warranties, express or implied, regarding the completeness, accuracy, reliability, suitability, or availability of the post, the website, or any information, products, services, or related graphics for any purpose. This content is not a substitute for professional medical advice, diagnosis, or treatment; always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition and never disregard or delay seeking professional advice because of something you have read here. Reliance on any information provided is solely at your own risk.

