Overview
Histamine is a ubiquitous biogenic amine that influences vascular tone and permeability, sensory nerve activity (especially itch), airway smooth muscle, gastric acid secretion, and central nervous system arousal. Among its diverse receptor subtypes (H1–H4), the H1 receptor is the dominant mediator of the hallmark symptoms of immediate-type (type I) allergic reactions in the skin and upper airways. Accordingly, the term “antihistamine” in everyday clinical usage refers to H1-receptor blockers, which are best described pharmacologically as inverse agonists that stabilize the inactive conformation of the H1 receptor.Core principles to ground the chapter:
- Histamine is synthesized from histidine by histidine decarboxylase (HDC), stored in mast cell and basophil granules, and released by immunologic (IgE cross-linking) and non-immunologic triggers.
- H1-receptor activation (Gq/11 → PLCβ → IP3/DAG → Ca2+) drives vasodilation, increased microvascular permeability (wheal), sensory nerve stimulation (itch, sneeze), and bronchoconstriction.
- Clinically used “antihistamines” are H1 inverse agonists that reduce receptor constitutive activity and block histamine effects; they differ substantially in CNS penetration, anticholinergic properties, duration, and interaction profiles.
- First-generation H1 antihistamines are effective but sedating and anticholinergic; second-generation agents retain peripheral efficacy with minimal CNS effects.
Because H1 pharmacology intersects with immunology, dermatology, otolaryngology, and respiratory medicine, understanding histamine biology and the pharmacokinetic/pharmacodynamic nuances of H1 antihistamines is central to rational therapy for allergic rhinitis, urticaria, pruritus, and related conditions.
Historical perspective
Shortly after histamine’s identification in the early 20th century, investigators described the “triple response of Lewis” following intradermal histamine or mechanical stimulation: a red spot (capillary dilation), surrounding flare (axon reflex vasodilation), and a wheal (edema from increased venular permeability). These observations linked histamine to the cardinal signs of immediate allergic skin responses. The first clinical H1 antagonists entered practice in the 1940s–1950s, providing notable relief of hay fever and hives, but with prominent sedation and anticholinergic adverse effects due to central H1 and off-target receptor blockade. “Second-generation” H1 antihistamines were designed decades later to minimize blood–brain barrier penetration and off-target actions, preserving anti-allergic efficacy while reducing drowsiness and cognitive impairment. The withdrawal of the older second-generation agents terfenadine and astemizole for torsadogenic interactions in the 1990s catalyzed a greater focus on cardiac safety and metabolic liabilities in newer drugs.
Chemistry, biosynthesis, storage, and degradation of histamine
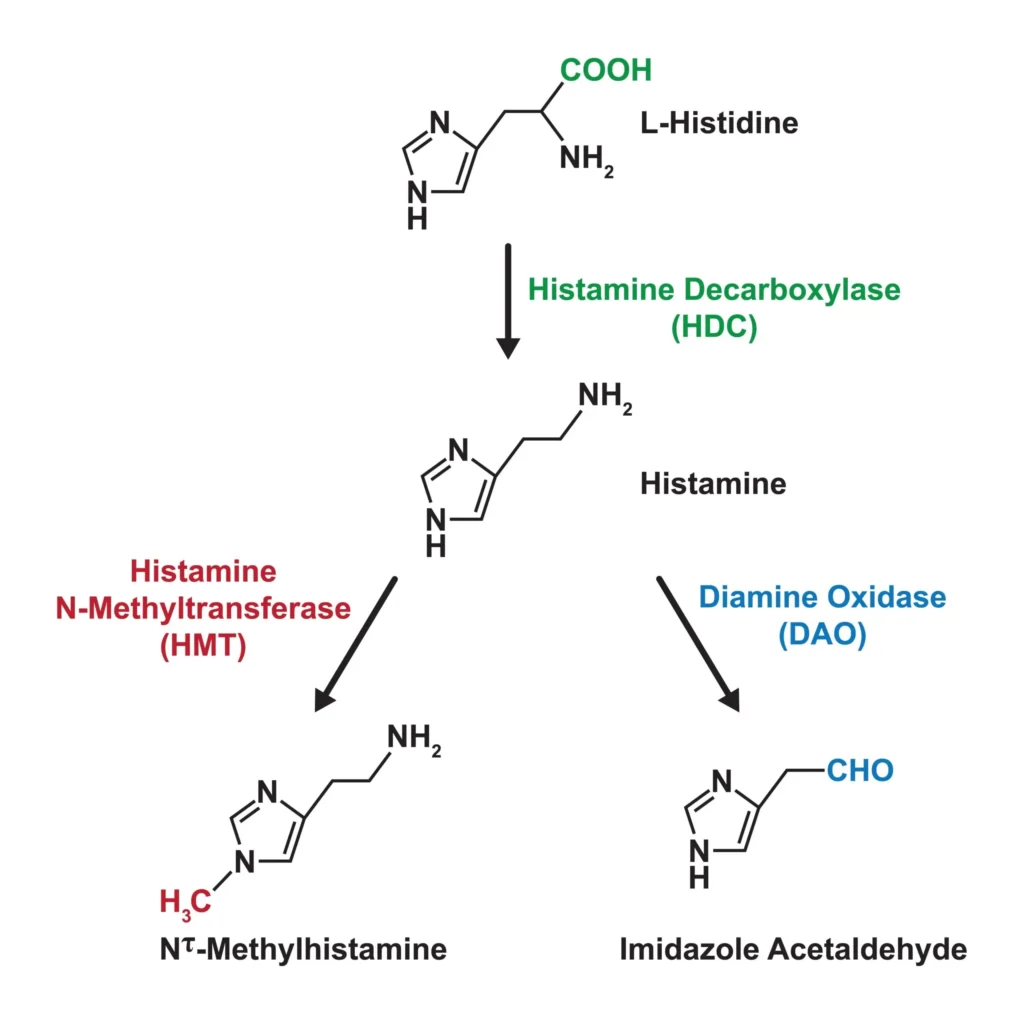
- Chemical structure: Histamine is 2-(4-imidazolyl)ethylamine. The imidazole ring (pKa ~5.8) and the terminal amine (pKa ~9.8) confer amphiprotic behavior and facilitate electrostatic interactions with proteoglycans such as heparin within acidic mast cell granules.
- Biosynthesis: Histidine decarboxylase (HDC), a pyridoxal phosphate–dependent enzyme, decarboxylates L-histidine to histamine. HDC expression is upregulated by cytokines (e.g., IL‑3, IL‑4, IL‑9) during mast cell/basophil differentiation and by gastrin in enterochromaffin-like cells. In neurons of the tuberomammillary nucleus, histamine functions as a neurotransmitter.
- Storage and release:
- In mast cells and basophils, histamine accumulates in secretory granules complexed with acidic proteoglycans along with tryptase, chymase, and other mediators.
- IgE-dependent degranulation: Allergen cross-linking of FcεRI-bound IgE activates Lyn/Syk → PLCγ → IP3/DAG → Ca2+ influx and cytoskeletal reorganization culminating in exocytosis. Released mediators include histamine and lipid mediators (LTs, PGs).
- Non-IgE triggers: Anaphylatoxins (C3a, C5a), neuropeptides (substance P), direct mast cell activators (e.g., radiocontrast, opioids like morphine), and physical stimuli (cold, vibration) can provoke release.
- Metabolism: Diamine oxidase (DAO; “histaminase”) and histamine-N-methyltransferase (HNMT) are the principal degradative enzymes. DAO predominates extracellularly (e.g., gut), whereas HNMT is important intracellularly (e.g., in CNS and liver). Genetic or acquired reductions in DAO/HNMT activity may contribute to histamine intolerance phenotypes in some patients.
The H1 receptor: signaling, distribution, and pathophysiologic roles

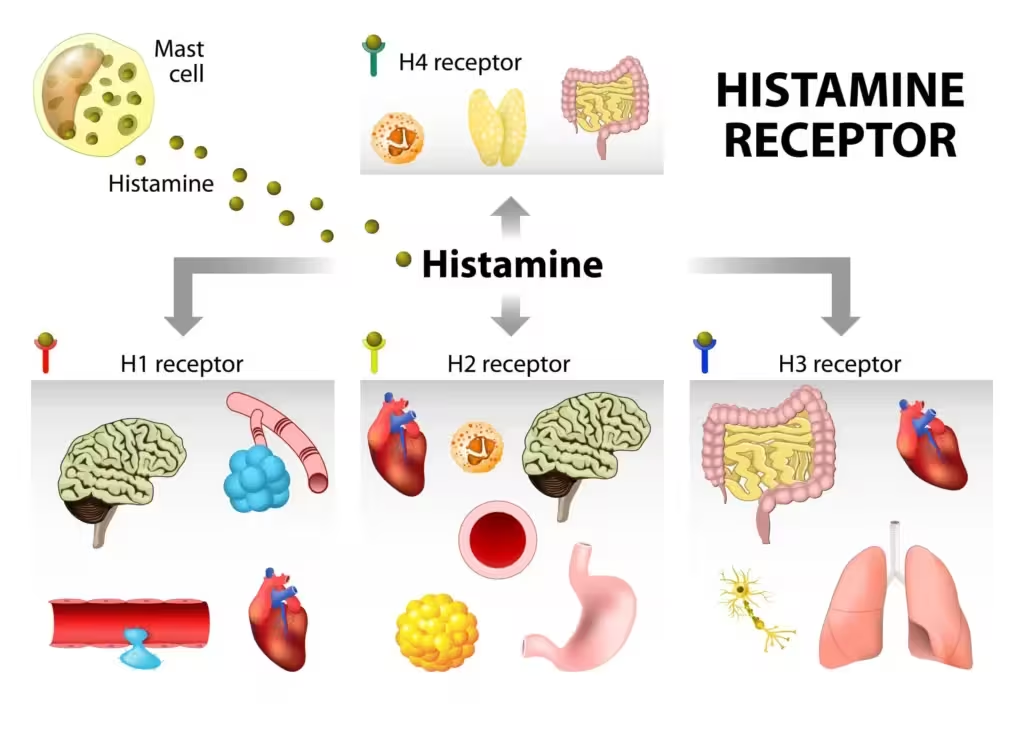
- Signal transduction: H1 is a class A GPCR primarily coupled to Gq/11. Activation leads to PLCβ stimulation, IP3-mediated Ca2+ mobilization, DAG-mediated PKC activation, and downstream nitric oxide synthase (eNOS) activation in endothelium. The receptor exhibits some constitutive activity, a key reason H1 “antagonists” behave as inverse agonists.
- Tissue distribution and actions:
- Endothelium/vasculature: H1 activation on endothelial cells causes NO-dependent vasodilation and cytoskeletal contraction with gap formation, increasing venular permeability and plasma extravasation. Clinically this yields erythema (flush) and edema (wheal).
- Peripheral sensory nerves: Stimulation of C-fiber pruriceptors drives itch and contributes to the flare via axon reflex vasodilation.
- Airways: H1 stimulation can cause bronchoconstriction and mucous secretion; histamine is one of many mediators in asthma, explaining why H1 blockers are not primary bronchodilators in chronic management.
- Skin: Urticarial wheals reflect transient dermal edema from increased permeability; angioedema involves deeper dermal/submucosal layers.
- CNS: Histamine from the tuberomammillary nucleus promotes wakefulness and attention through H1 receptors; blockade underlies sedation and cognitive slowing with first-generation agents.
- Clinical phenomena: Dermatographism, cold and cholinergic urticaria, acute urticaria from infections, and allergic rhinitis exemplify conditions where H1-mediated histamine effects are central. Anaphylaxis includes multi-mediator cascades; H1 blockade helps cutaneous and some GI symptoms but is adjunctive to epinephrine.
Measuring histamine-mediated activity and related syndromes
- Laboratory indices: Plasma histamine is transient; urine N-methylhistamine and serum tryptase (mast cell marker) are often more practical. DAO activity assays are used by some for suspected histamine intolerance, but clinical utility and standardization are limited.
- Mast cell activation disorders: Recurrent anaphylactoid episodes with elevated tryptase support mast cell activation syndrome; systemic mastocytosis (often KIT mutations) features flushing, urticaria pigmentosa, GI symptoms, and anaphylaxis risk.
- Scombroid poisoning: Spoiled fish with high histamine produces flushing, headache, wheals, bronchospasm, and GI upset; H1 antihistamines often provide rapid symptom relief.
- Histamine intolerance: A debated construct positing impaired degradation (e.g., low DAO) with symptoms after histamine-rich foods; management often empirical, including dietary adjustment and H1 blockers for symptomatic relief.
What “antihistamine” means in practice: focus on H1 inverse agonists
- Mechanistic definition: Most approved agents are inverse agonists at H1 receptors, reducing basal activity and competitively antagonizing histamine. This yields attenuation of vasodilation/permeability and sensory nerve activation, translating to reduced itch, sneezing, and tearing.
- First vs second generation:
- First-generation H1 antihistamines are lipophilic, cross the blood–brain barrier, and commonly antagonize muscarinic, serotonergic, and α-adrenergic receptors and block sodium channels. They are effective but sedating, with anticholinergic adverse effects and greater overdose toxicity.
- Second-generation H1 antihistamines are more polar and/or P‑glycoprotein substrates, limiting CNS penetration. They provide similar peripheral efficacy with much lower rates of sedation and anticholinergic effects.
Key clinical implication: For routine allergic rhinitis and chronic urticaria, second-generation H1 antihistamines are preferred. First-generation agents retain roles when sedation or antimuscarinic effects are desired (e.g., nocturnal pruritus, motion sickness), but should be used judiciously, especially in older adults.
Structure–activity relationships and selectivity
- Shared pharmacophore: Most H1 blockers possess a diaryl (or aryl-heteroaryl) motif linked via a short spacer to a tertiary amine. Protonation at physiological pH promotes receptor binding.
- CNS penetration: Lipophilicity and absence of active efflux enhance BBB entry and sedation. Second-generation drugs are less lipophilic and/or are P-gp substrates, limiting brain exposure.
- Off-target actions: First-generation agents display meaningful affinity for muscarinic (M1), serotonergic (5-HT2), and α1-adrenergic receptors and voltage-gated sodium channels—mechanisms underlying anticholinergic effects, orthostatic symptoms, antiemesis, and local anesthetic activity.
Classification and representative agents
First-generation H1 antihistamines (sedating):
- Ethanolamines: diphenhydramine, doxylamine, carbinoxamine.
- Alkylamines: chlorpheniramine, brompheniramine, dexchlorpheniramine.
- Ethylenediamines: tripelennamine (historical).
- Piperazines: hydroxyzine, cyclizine, meclizine.
- Phenothiazines/tricyclics: promethazine; doxepin (a tricyclic with potent H1 blockade).
- Key characteristics: potent H1 antagonism with variable antimuscarinic/antiemetic properties; high sedation/cognitive impairment risk; useful for motion sickness (meclizine, dimenhydrinate), nausea (promethazine), and nocturnal itch (hydroxyzine, doxepin).
Second-generation H1 antihistamines (minimally sedating):
- Piperidines: loratadine → desloratadine (active metabolite); fexofenadine; bilastine; rupatadine; ebastine; mizolastine.
- Piperazines: cetirizine; levocetirizine (active enantiomer).
- Others/topicals: azelastine (intranasal/ophthalmic); olopatadine (intranasal/ophthalmic); ketotifen and bepotastine (ophthalmic; ketotifen also oral in some markets).
- Key characteristics: similar efficacy across class for allergic rhinitis/urticaria; low sedation at standard doses; longer duration allows once-daily dosing for many agents.
Note on terminology: Marketing sometimes labels active metabolites/enantiomers (e.g., desloratadine, levocetirizine) as “third-generation,” but pharmacologically they are refined second-generation agents.
Pharmacokinetics and practical considerations
- Onset and duration:
- Oral second-generation agents typically begin relief within 1–3 hours (faster for cetirizine, levocetirizine; somewhat slower for loratadine/desloratadine) and last ~24 hours.
- Intranasal antihistamines (azelastine, olopatadine) have rapid onset (often within 15–30 minutes), helpful for breakthrough nasal symptoms.
- Ophthalmic formulations act quickly for ocular itch and tearing.
- Absorption and food effects:
- Bilastine: absorption reduced with food/fruit juice; administer on an empty stomach (e.g., 1 hour before or 2 hours after meals).
- Fexofenadine: bioavailability reduced by grapefruit, orange, and apple juices via OATP inhibition; separate by several hours and avoid coadministration with juices.
- Cetirizine/levocetirizine: minimal food effect; rapid onset.
- Distribution:
- First-generation agents distribute widely, including the CNS; second-generation agents have limited CNS distribution.
- Metabolism and elimination:
- Loratadine is extensively metabolized (CYP3A4/2D6) to desloratadine (active). Desloratadine undergoes CYP3A4/2C8 metabolism; long half-life supports once-daily dosing.
- Cetirizine is minimally metabolized and primarily renally excreted; dose adjustments in renal impairment.
- Levocetirizine is renally cleared; adjust in renal dysfunction.
- Fexofenadine is mostly excreted unchanged via feces and urine; substrate of P‑gp and OATP.
- Hydroxyzine undergoes hepatic metabolism, partly to cetirizine.
- Ebastine/mizolastine and rupatadine undergo hepatic metabolism (CYP3A4 involvement for several).
- Pharmacogenomics may influence exposure for some agents, but routine testing is not indicated.
- Half-lives and dosing frequency:
- Many second-generation agents support once-daily dosing; acrivastine typically requires multiple daily doses.
- First-generation agents vary widely; many require dosing every 4–6 hours for sustained effect.
Clinical efficacy: what H1 antihistamines do well
- Allergic rhinitis (seasonal/perennial):
- Reduce sneezing, rhinorrhea, nasal/ocular itch, and tearing. Less effective for nasal congestion than intranasal corticosteroids or decongestants.
- Second-generation oral H1 antagonists are first-line for mild disease and adjunctive to intranasal steroids in moderate–severe disease.
- Intranasal antihistamines (e.g., azelastine) offer rapid symptom relief and can meaningfully reduce congestion; combination sprays (antihistamine + steroid) often outperform either alone.
- Allergic conjunctivitis:
- Ophthalmic antihistamines with mast cell–stabilizing properties (olopatadine, ketotifen, bepotastine) provide rapid itch relief and prophylaxis with regular use.
- Urticaria:
- Acute urticaria: Oral second-generation agents effectively reduce wheals and itch.
- Chronic spontaneous urticaria (CSU): Daily second-generation H1 antihistamines are first-line. When standard dosing is inadequate, guideline-supported “up-dosing” up to fourfold is commonly used before progressing to biologics (e.g., anti-IgE) or immunomodulators. Sedating first-generation drugs may be considered at night for severe nocturnal itch, balancing risks.
- Pruritus from dermatologic/systemic causes:
- Effective when histamine is a dominant mediator (e.g., urticaria, insect bites). For pruritus with other pathways (e.g., cholestatic, uremic), H1 blockers often provide limited relief; sedating agents can still help sleep.
- Anaphylaxis (adjunctive only):
- H1 antihistamines ameliorate cutaneous symptoms and may reduce some gastrointestinal manifestations; they do not prevent airway compromise or shock. Epinephrine is the lifesaving first-line therapy.
- Motion sickness and vestibular nausea:
- First-generation agents (meclizine, dimenhydrinate, promethazine) reduce motion-induced nausea and vertigo through combined H1 and antimuscarinic activity; best when taken prophylactically.
- Insomnia (short-term symptomatic use):
- Diphenhydramine and doxylamine can induce sleep but often cause next-day sedation and anticholinergic effects. They are not preferred for chronic insomnia, especially in older adults.
- Common cold symptom relief:
- First-generation agents can modestly reduce rhinorrhea and sneezing through anticholinergic drying and H1 blockade; second-generation agents have less benefit in viral URIs.
What H1 antihistamines do not do
- They are not decongestants. Nasal congestion is less responsive to H1 blockade; intranasal steroids or short-term decongestants are more effective.
- They are not bronchodilators. H1 blockade plays only a limited role in asthma; inhaled bronchodilators and anti-inflammatory agents are standard.
- They do not reverse anaphylactic hypotension/airway edema. Epinephrine, airway support, and IV fluids are vital; H1 antagonists are supportive for skin/GI symptoms.
Safety and tolerability
- Sedation and psychomotor impairment:
- Common with first-generation agents; interindividual variability is high. Effects include slowed reaction time and impaired driving. Patients should be counseled explicitly about machinery/vehicle operation.
- Second-generation drugs have low sedation risk at standard doses; cetirizine/levocetirizine can cause mild drowsiness in a subset of patients.
- Anticholinergic burden (first-generation):
- Dry mouth, blurred vision, constipation, urinary retention; delirium and cognitive impairment in older adults. Strongly discouraged in geriatrics by Beers criteria unless no safer alternative exists.
- Cardiac safety:
- Historic torsades risk with terfenadine/astemizole in the presence of CYP3A4 inhibitors led to withdrawal; modern agents like fexofenadine, cetirizine, loratadine, and desloratadine have favorable cardiac profiles at recommended doses.
- Hydroxyzine carries QT prolongation warnings in patients with risk factors; use lowest effective dose and avoid co-prescribing with other QT-prolonging drugs in at-risk individuals.
- Paradoxical CNS effects:
- Children may exhibit excitation, irritability, or insomnia rather than sedation.
- Local/dermatologic reactions:
- Topical diphenhydramine can cause allergic contact dermatitis and is generally discouraged.
- Doxepin cream (5%) is effective for localized pruritus but may cause systemic drowsiness due to absorption.
- GI and other effects:
- Nausea, dyspepsia, and headache occur uncommonly. First-generation agents can lower seizure threshold at high doses or in predisposed patients.
Drug–drug and drug–food interactions
- CNS depressants: Additive sedation with alcohol, benzodiazepines, opioids, sedative-hypnotics; advise against concurrent use, especially with first-generation agents.
- CYP-mediated interactions:
- Loratadine/desloratadine exposures can increase with strong CYP3A4 inhibitors; clinically significant events are uncommon at standard doses but monitor as needed.
- Hydroxyzine levels may increase with CYP inhibitors; consider dose reductions in polypharmacy.
- Transporter/food interactions:
- Fexofenadine: reduced absorption with grapefruit/orange/apple juices (OATP inhibition); separate by 2–4 hours and avoid juices near dosing.
- Bilastine: decreased absorption with food and grapefruit; administer away from meals.
- QT-prolonging co-medications: Exercise caution combining any antihistamine with potent QT-prolonging drugs, particularly in patients with risk factors (electrolyte abnormalities, congenital LQTS, structural heart disease).
Dosing strategies and formulations
- General dosing:
- Most second-generation antihistamines are dosed once daily; consistency is important for chronic conditions like perennial allergic rhinitis and CSU.
- In CSU, dose escalation of a second-generation agent up to 4× the standard dose is commonly recommended before adding or switching therapies.
- Formulations:
- Oral tablets/capsules, orodispersible tablets, syrups for pediatrics, extended-release combinations with decongestants (e.g., loratadine–pseudoephedrine).
- Intranasal sprays: azelastine and olopatadine; combination steroid–antihistamine sprays are effective for moderate–severe rhinitis.
- Ophthalmic drops: ketotifen, olopatadine, bepotastine; often dosed once or twice daily.
- Practical pearls:
- For rapid nasal symptom relief, intranasal antihistamines offer faster onset than oral agents.
- For predominantly ocular symptoms, topical ophthalmic formulations provide targeted benefit and reduce systemic exposure.
- Counsel about bitter taste with intranasal azelastine; proper technique (slight head tilt forward, avoid sniffing hard) can reduce drip and taste.
Special populations
- Pediatrics:
- Avoid sedating first-generation antihistamines when possible; paradoxical excitation can occur.
- Age-specific dosing limits vary by product; verify labeling carefully. For motion sickness, meclizine is not approved for very young children; nonpharmacologic strategies are preferred for toddlers.
- For allergic rhinitis and urticaria, cetirizine, levocetirizine, loratadine, and desloratadine have pediatric formulations with established dosing.
- Geriatrics:
- Minimize or avoid first-generation agents due to anticholinergic cognitive burden, urinary retention, glaucoma precipitation, constipation, and fall risk. Second-generation agents are preferred.
- Start low and titrate cautiously; monitor for sedation even with second-generation agents.
- Pregnancy and lactation:
- Agents with long safety records (chlorpheniramine among older drugs; loratadine and cetirizine among second-generation agents) are commonly chosen when medication is necessary.
- Hydroxyzine is often avoided during the first trimester. Always individualize decisions and consult current references.
- Many antihistamines are excreted in breast milk; monitor infants for irritability or sedation if maternal therapy is needed.
- Renal/hepatic impairment:
- Cetirizine/levocetirizine and famotidine (H2 blocker, not an H1 antihistamine) are renally cleared; for H1 practice, ensure renal dose adjustments for cetirizine/levocetirizine.
- Loratadine/desloratadine rely on hepatic metabolism; use caution in significant hepatic impairment.
- Occupational safety:
- Drivers, pilots, and machine operators should avoid sedating antihistamines; documentation of non-impairing choices (e.g., fexofenadine) is prudent in safety-sensitive roles.
Overdose and toxicity
- First-generation antihistamine overdose:
- Presents with anticholinergic toxidrome (hyperthermia, mydriasis, dry mucosa, tachycardia, urinary retention), agitation/delirium, seizures. Cardiotoxicity can include QRS widening (Na+ channel block) and QT prolongation at very high levels.
- Management: airway/ventilation as needed; benzodiazepines for agitation/seizures; sodium bicarbonate for wide QRS; cooling for hyperthermia; physostigmine is reserved for severe, pure anticholinergic delirium with careful ECG monitoring and readiness to treat bradyarrhythmias.
- Second-generation overdose:
- Usually milder (somnolence, tachycardia, GI upset). Serious arrhythmias are rare at therapeutic doses; monitor if co-ingestants include QT-prolonging or CYP-inhibiting agents.
Clinical selection: matching the antihistamine to the patient
- Allergic rhinitis:
- Mild intermittent: oral second-generation H1 (e.g., cetirizine, loratadine, fexofenadine); consider intranasal antihistamine for rapid onset or if congestion prominent, or use a combination spray (steroid + antihistamine) for broader control.
- Persistent or moderate–severe: intranasal corticosteroid is foundational; add oral or intranasal H1 antihistamine for residual itch/sneeze/tearing.
- Chronic spontaneous urticaria:
- Start with once-daily second-generation H1; escalate dose (up to 4× standard) if needed; consider nighttime addition of a sedating agent only if insomnia from itch is severe and risks are acceptable. If refractory, move to advanced therapies per specialist guidance.
- Predominant ocular symptoms:
- Ophthalmic antihistamine/mast cell stabilizers are preferred for rapid, targeted relief.
- Nocturnal pruritus:
- A sedating agent (e.g., hydroxyzine, doxepin) can be considered at night; reassess frequently given anticholinergic burden and next-day impairment.
- Motion sickness/vestibular symptoms:
- Use meclizine or dimenhydrinate prophylactically; consider adding or substituting antimuscarinic therapy depending on severity and contraindications.
- Patients at high risk of sedation or falls:
- Prefer fexofenadine, loratadine, or desloratadine; avoid first-generation agents. Cetirizine/levocetirizine may still cause mild drowsiness in some.
- Polypharmacy/CYP interactions:
- Favor agents with minimal metabolism and interaction risk (e.g., cetirizine, levocetirizine, fexofenadine). Counsel on food–drug interactions (juices with fexofenadine; meals with bilastine).
Controversies and clinical nuances
- Are all second-generation antihistamines equivalent?
- Across populations, efficacy for allergic rhinitis and urticaria is broadly similar, though individuals vary in response. Cetirizine/levocetirizine are sometimes perceived as slightly more potent for urticaria but may cause drowsiness in a subset; fexofenadine is among the least sedating and has minimal cardiac/CNS risk but is sensitive to juice interactions; loratadine/desloratadine are well tolerated and once daily.
- Tolerance and “antihistamine failure”:
- True pharmacodynamic tachyphylaxis to H1 blockade appears limited; symptom fluctuations are more often due to variable allergen load, inflammatory cofactors, or inadequate dosing. For CSU, up-dosing is preferred over switching classes repeatedly.
- Combining multiple H1 antihistamines:
- Routine combination of two different second-generation H1 agents is generally not recommended; up-dosing a single second-generation agent has more evidence in CSU. Targeted addition of intranasal or ophthalmic antihistamines can be beneficial for local symptoms.
- Atopic dermatitis:
- H1 antihistamines do not alter the underlying dermatitis but can aid sleep by reducing itch perception; sedating options may be considered short term at night.
- Use of first-generation antihistamines in children:
- Despite OTC availability, they are not benign; paradoxical excitation and anticholinergic effects are common. Modern guidelines favor second-generation agents for pediatric allergic conditions.
Future directions in H1 antihistamine therapy
- Biased ligands and allosteric modulators: Molecules that preferentially modulate H1 signaling pathways (e.g., pruritus suppression without vascular effects) could refine the benefit–risk balance.
- Dual-acting agents: Compounds with H1 antagonism plus additional actions (e.g., platelet-activating factor antagonism with rupatadine; mast cell stabilization with ketotifen/olopatadine) illustrate an evolving strategy to broaden symptom control.
- Novel delivery systems: Enhanced intranasal/ophthalmic formulations and sustained-release matrices may improve adherence and onset profiles.
- Precision medicine: Better phenotyping of pruritus and urticaria endotypes may guide choice and dosing of H1 blockers or earlier transition to adjunctive biologics where histamine is not the dominant mediator.
Practical counseling points for patients
- Set expectations: Antihistamines work best for itch, sneeze, and runny nose; they do less for congestion.
- Safety first: Avoid alcohol and sedatives with first-generation agents; do not drive or operate machinery until you know how the medicine affects you—even “non-drowsy” drugs can rarely cause sedation.
- Dosing consistency: Daily dosing works better than as-needed for persistent symptoms; escalate under guidance for chronic hives.
- Food interactions: Do not take fexofenadine with fruit juices; take bilastine on an empty stomach.
- When to seek help: If hives persist despite high-dose second-generation therapy, or if you experience breathing difficulty, throat tightness, or dizziness/lightheadedness suggestive of anaphylaxis, seek urgent care.
Key takeaways
- In routine clinical usage, “antihistamines” means H1-receptor blockers.
- H1 activation drives vasodilation, vascular permeability, itch, and sneezing—hallmark allergic symptoms that H1 antihistamines effectively blunt.
- First-generation agents are effective but sedating and anticholinergic; second-generation agents provide comparable peripheral efficacy with far fewer CNS effects and are preferred for most patients.
- Selecting the right formulation (oral vs intranasal vs ophthalmic), dosing strategy (daily vs up-dosing in CSU), and counseling on interactions and sedation risks are central to optimal outcomes.
References
- Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 14th ed. Brunton LL, Hilal‑Dandan R, Knollmann BC, eds. McGraw‑Hill Education; 2023. Chapters: Histamine and Antihistamines; Pharmacotherapy of Allergic Disorders.
- Katzung & Trevor’s Basic & Clinical Pharmacology. 16th ed (with 2023 updates). Katzung BG, Kruidering‑Hall M, Trevor AJ, eds. McGraw‑Hill Education; 2021–2023. Sections: Histamine, Serotonin, and Ergot Alkaloids; H1‑Antihistamines.
- Rang & Dale’s Pharmacology. 9th ed. Rang HP, Ritter JM, Flower RJ, Henderson G, eds. Elsevier; 2019–2020. Chapters: Autacoids and Inflammation; Histamine and 5‑HT; Drugs Used in Allergy.
- Middleton’s Allergy: Principles and Practice. 9th ed. Burks AW, Holgate ST, O’Hehir RE, Bacharier LB, Broide DH, Hershey GKK, Peebles RS Jr, eds. Elsevier; 2020 (with online updates). Sections: Pharmacology of Antihistamines; Management of Allergic Rhinitis and Urticaria.
- Robbins & Cotran Pathologic Basis of Disease. 10th ed. Kumar V, Abbas AK, Aster JC. Elsevier; 2020. Chapters: Acute and Chronic Inflammation; Hypersensitivity Reactions.
- Pharmacotherapy: A Pathophysiologic Approach. 12th ed. DiPiro JT, Yee GC, Posey LM, Haines ST, Nolin TD, Ellingrod VL, eds. McGraw‑Hill Education; 2023. Chapters: Allergic Rhinitis; Urticaria and Angioedema; Anaphylaxis—Acute Management (adjunctive role of H1 antihistamines).
- Brody’s Human Pharmacology: Molecular to Clinical. 6th ed. Wecker L, Crespo LM, Dunaway G, Faingold CL, Watts S, eds. Elsevier; 2018–2020. Sections: Autacoids; Histamine Metabolism; H1‑Receptor Pharmacology.
- Clinical Pharmacy and Therapeutics. 6th ed. Walker R, Whittlesea C, eds. Elsevier; 2018–2020 updates. Sections: Respiratory and Allergy—Pharmacologic Management; Urticaria and Pruritus—Role of H1 Antihistamines.
Quiz on Antihistamines
Quiz on Antihistamines
Medical Disclaimer
The medical information on this post is for general educational purposes only and is provided by Pharmacology Mentor. While we strive to keep content current and accurate, Pharmacology Mentor makes no representations or warranties, express or implied, regarding the completeness, accuracy, reliability, suitability, or availability of the post, the website, or any information, products, services, or related graphics for any purpose. This content is not a substitute for professional medical advice, diagnosis, or treatment; always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition and never disregard or delay seeking professional advice because of something you have read here. Reliance on any information provided is solely at your own risk.

