Introduction
Histamine stands as a fundamental mediator in allergic reactions, inflammatory processes, and various physiological functions such as gastric acid secretion and neurotransmission. Endogenously, it is produced and stored predominantly in mast cells and basophils, with additional roles in neurons. When released, histamine exerts significant effects on smooth muscles, vascular endothelium, and secretory tissues, orchestrating symptoms associated with allergic responses (e.g., urticaria, bronchoconstriction, hypotension). The discovery of antihistamines—drugs that target histamine receptors—has radically improved the management of allergies, motion sickness, and other histamine-related conditions.
Over the past decades, understanding of histamine’s pharmacology and receptor subtypes has expanded. Notably, the development of second-generation antihistamines addressed sedation and anticholinergic effects observed with earlier compounds. Researchers have also explored histamine’s immunomodulatory and neuromodulatory roles, offering novel therapeutic implications. This article offers an in-depth analysis of histamine’s synthesis, storage, release mechanisms, receptor subtypes, and pathophysiological impacts. It further examines the pharmacology of antihistamines, distinguishing first-generation from newer-generation agents, their clinical applications, adverse effect profiles, and future directions in the field.
Histamine Overview
Biosynthesis of Histamine
Histamine is synthesized via decarboxylation of the amino acid histidine, catalyzed by the enzyme histidine decarboxylase (HDC). Mast cells and basophils represent primary sources, storing histamine in secretory granules complexed with proteoglycans (e.g., heparin). In addition, certain neurons produce histamine for neurotransmission, particularly in the hypothalamus (tuberomammillary nucleus), modulating wakefulness and appetite.
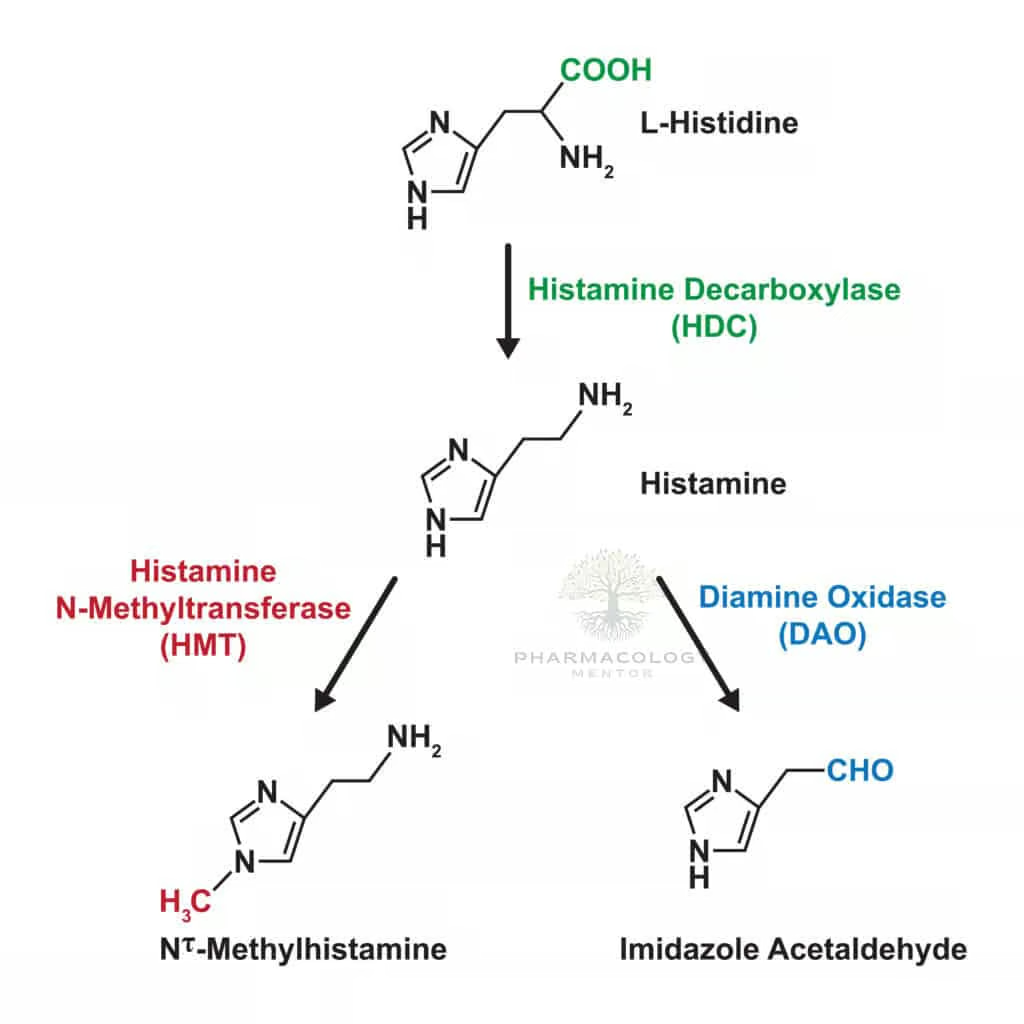
Storage and Release
Once formed, histamine is stored in vesicles until triggered by immunologic or non-immunologic stimuli. Immunologic release typically involves IgE antibodies binding antigens on mast cells or basophils, prompting degranulation via cross-linking of IgE receptors (FcεRI). Non-immunologic triggers—physical stimuli like temperature change, chemicals such as morphine, or some antibiotics—can also release histamine. In pathophysiologic states, massive histamine release underlies systemic reactions (anaphylaxis) or localized allergic responses (rhinitis, dermatitis).
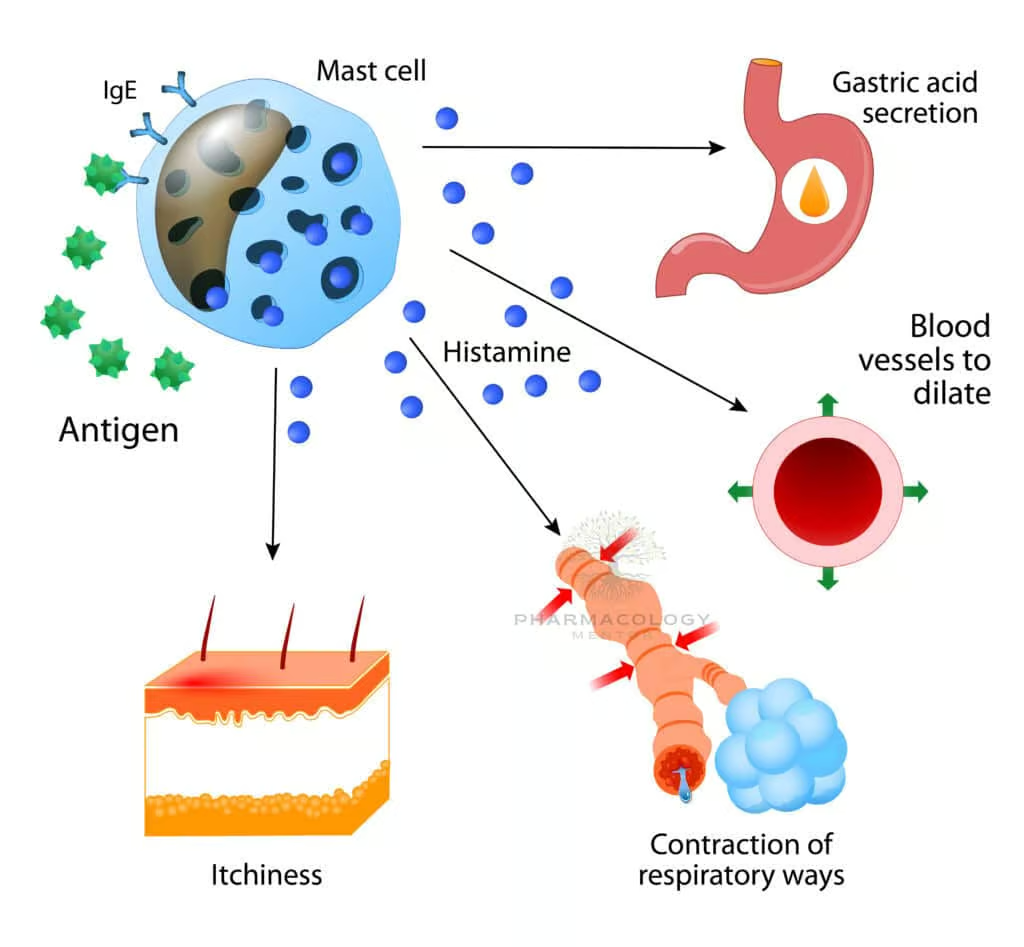
Physiological and Pathophysiological Roles
Histamine influences numerous bodily systems:
- Allergic and Immunologic Reactions: Vasodilation, increased vascular permeability, pruritus, bronchoconstriction.
- Gastric Acid Secretion: Histamine acts on H2 receptors in parietal cells, stimulating acid release.
- Neurotransmission: Central histamine modulates alertness, energy balance, and cognition.
- Vascular Tone and Permeability: Endothelial cells respond to histamine by forming gaps, enabling plasma proteins to extravasate and cause edema.
Despite histamine’s necessity for normal physiology, its dysregulated release leads to allergic manifestations, anaphylaxis, or chronic inflammation in susceptible individuals.
Histamine Receptors
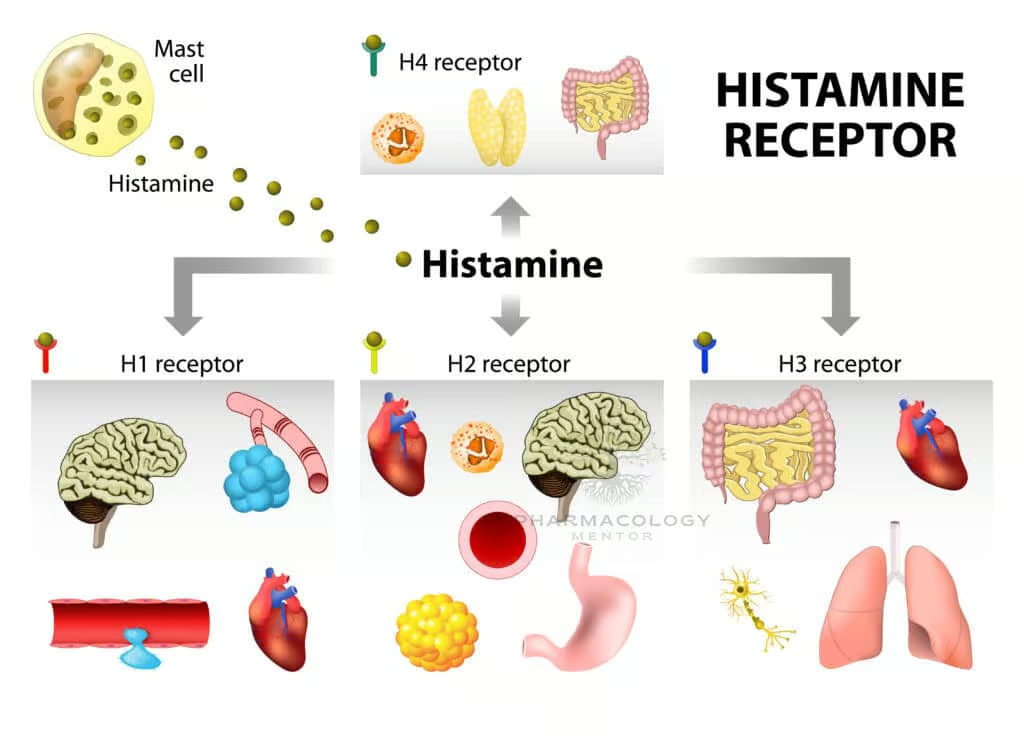

Classification
Four known histamine receptor subtypes—H1, H2, H3, and H4—are G-protein-coupled receptors (GPCRs) with different localization and functional outcomes:
- H1 Receptor: Gq/11-coupled in smooth muscle, endothelium, CNS. Activation triggers phospholipase C (PLC), increasing intracellular calcium, leading to vasodilation, increased permeability, and bronchoconstriction. Centrally, H1 activity regulates wakefulness.
- H2 Receptor: Gs-coupled primarily in parietal cells (acid secretion) and cardiomyocytes, stimulating adenylate cyclase and raising cAMP levels. Effects include increased gastric acid secretion and modest positive chronotropic and inotropic cardiac actions.
- H3 Receptor: Gi/o-coupled, mostly in presynaptic neurons. Acts as an autoreceptor/heteroreceptor regulating histamine and other neurotransmitter releases. H3 influences sleep-wake cycles, appetite, and cognition.
- H4 Receptor: Gi/o-coupled; expressed on immune cells (mast cells, eosinophils, T cells). Implicated in chemotaxis and immune response modulation, representing emerging targets for inflammatory and pruritic conditions.
H1 Receptor and Allergy
The bulk of allergic phenomena (rashes, edema, pruritus) are mediated by H1 receptor activation on vascular endothelium and sensory nerve endings. This receptor’s significance guides the utility of H1 antihistamines in clinical practice for acute allergic responses.
H2 in Gastric Secretion and Cardiac Effects
H2 receptor stimulation on parietal cells elevates proton pump activity, boosting gastric acid secretion. Blocking these receptors underlies the mechanism of H2 receptor antagonists (e.g., cimetidine) used in peptic ulcer disease or gastroesophageal reflux disease (GERD). H2 also modulates cardiac contractility and rate, though typically overshadowed by predominant adrenergic control.
Central H3 and Peripheral H4 Insights
H3 modulates CNS neurotransmitter release, influencing arousal, appetite, and memory. Preclinical data suggest H3 antagonists may promote wakefulness or cognition. Meanwhile, H4 is a potential therapeutic target in atopic dermatitis or pruritus, though clinical applications remain in early stages.
Pathophysiology of Histamine in Disease
Allergic Rhinitis and Urticaria
Histamine-driven vasodilation and increased permeability incite rhinorrhea, nasal congestion, pruritus, and sneezing in allergic rhinitis. In urticaria, histamine fosters wheal-and-flare responses, manifesting as hives. H1 antihistamines often suffice to control these symptoms, although severe cases (e.g., chronic urticaria) might require additional anti-inflammatory agents.
Anaphylaxis
A life-threatening, systemic IgE-mediated hypersensitivity reaction involves massive mast cell degranulation, releasing histamine and other mediators (leukotrienes, prostaglandins). Clinical features (hypotension, bronchospasm, angioedema) necessitate epinephrine as first-line therapy. Antihistamines, both H1 and H2 blockers, serve adjunct roles in alleviating cutaneous or GI manifestations.
Asthma and Atopic Dermatitis
Although histamine contributes to asthmatic bronchoconstriction, modern therapies focus more on inhaled corticosteroids and bronchodilators. Nonetheless, for some patients with allergic triggers, antihistamines can help. In atopic dermatitis, histamine-induced itch underpins the partial benefit from sedating antihistamines at night, reducing scratching behaviors.
Peptic Ulcer Disease
Histamine significantly regulates acid secretion via parietal cell H2 receptors. H2 receptor antagonists historically managed peptic ulcers or GERD, although widespread usage of proton pump inhibitors (PPIs) has eclipsed them in many clinical scenarios.
Antihistamines: General Principles
Mechanism of Action
Antihistamines primarily function by reversibly, competitively blocking histamine receptors, stopping histamine from exerting its physiological or pathophysiological effects. Most clinically used compounds target H1 or H2 receptors:
- H1 Antihistamines: Alleviate allergy symptoms (sneezing, itching, runny nose, watery eyes), reduce motion sickness, cause sedation in first-generation forms.
- H2 Antihistamines: Decrease gastric acid output, improving peptic ulcer and reflux symptoms.
Classification by Receptor Selectivity
- H1 Receptor Antagonists: Usually referred to as “antihistamines” in common parlance.
- H2 Receptor Antagonists: Often called “acid blockers” or “reducing acid secretion” agents.
- H3/H4 Antagonists: Largely under investigation; no widely used mainstream therapies exist yet in clinical practice, though potential future expansions are plausible.
Pharmacokinetics
Antihistamines vary in absorptive properties, lipophilicity, metabolism, and half-lives. First-generation H1 antagonists are more lipophilic, cross the blood-brain barrier, and undergo hepatic metabolism. Second-generation are more polar, exhibit lower brain penetration (hence less sedation), and have diverse elimination pathways. H2 antagonists are well absorbed orally, with significant hepatic and renal excretion.
Therapeutic Considerations
Antihistamines remain a mainstay for allergic disorders, but their choices hinge on sedation profiles, half-life, and potential drug interactions. Moreover, combination therapy with decongestants or leukotriene receptor antagonists can enhance efficacy in allergic rhinitis. For refractory chronic urticaria, higher than standard doses or conjunction with immunomodulators may be necessary.
H1 Antihistamines: Detailed Examination
First-Generation H1 Antihistamines
Examples include Diphenhydramine, Chlorpheniramine, Promethazine, Hydroxyzine, and Dimenhydrinate. They exhibit strong antagonism at H1 receptors but also block muscarinic receptors, alpha-adrenergic receptors, or serotonin receptors at varying degrees. The key characteristics include:
- Crossing the Blood-Brain Barrier: Sedation, drowsiness, or sedation-like euphoria can result. Consequently, some find them useful as OTC sleep aids, whereas sedation may be an unwanted effect for others.
- Anticholinergic Effects: Dry mouth, blurred vision, urinary retention, constipation.
- Motion Sickness: Dimenhydrinate and Promethazine are effective in preventing or treating motion sickness and postoperative nausea/vomiting.
- Shorter Duration: Typically require multiple daily doses, as half-lives can be shorter (adults: 4–12 hours).
First-generation antihistamines, despite sedation, remain beneficial for acute allergic responses demanding immediate relief. Caution is indicated in patients with glaucoma, prostatic hypertrophy, or those needing psychomotor performance (e.g., driving).
Second-Generation H1 Antihistamines
Examples include Cetirizine, Loratadine, Fexofenadine, Desloratadine, and Levocetirizine. Designed to minimize CNS penetration, they present fewer sedative effects. Key benefits include:
- Non-Sedating or Low-Sedating: Ideal for daytime use in allergic rhinitis or chronic urticaria without impairing alertness.
- Longer Half-Lives: Once-daily dosing fosters better compliance.
- Reduced Anticholinergic Effects: Minimal dryness or constipation.
- High Specificity: Primarily block peripheral H1 sites.
These agents revolutionized allergy treatment, offering effective symptom relief with minimal sedation. Some, like Cetirizine, can still induce mild sedation in sensitive individuals. Moreover, second-generation antihistamines have fewer interactions with hepatic enzymes or other drugs, though caution remains prudent.
Third-Generation H1 Antihistamines
“Third-generation” is sometimes used to describe active metabolites or enantiomerically refined versions of second-generation agents, purportedly with even fewer side effects and improved pharmacokinetics. Examples often cited include Levocetirizine (the active enantiomer of cetirizine) or Desloratadine (active metabolite of loratadine). While the nomenclature can be marketing-driven, these agents may offer small incremental benefits in potency or reduced sedation compared to their parent molecules.
Clinical Uses of H1 Antihistamines
Allergic Rhinitis, Conjunctivitis, and Urticaria
In mild to moderate allergic rhinitis, second-generation H1 antihistamines are first-line therapy. They efficiently reduce sneezing, rhinorrhea, and itching but have a limited effect on nasal congestion (where nasal steroids or decongestants help). Chronic urticaria responds well to up-dosed second-generation agents, sometimes up to fourfold standard dosage. First-generation antihistamines can be used at bedtime for sedation or refractory itching relief.
Atopic Dermatitis and Pruritus
Though the fundamental inflammation in atopic dermatitis involves complex immune dysregulation, H1 antihistamines may ease pruritus. Sedating antihistamines (e.g., Hydroxyzine) at night can mitigate scratch-induced lesions by promoting sedation. Non-sedating forms are also beneficial, albeit less so for intense pruritic episodes.
Motion Sickness and Vestibular Disturbances
Dimenhydrinate, Meclizine, Cyclizine, and Promethazine stand out for prophylaxis and management of motion sickness. Their anticholinergic actions in CNS vestibular pathways help limit nausea and vomiting. Promethazine is also used for postoperative or drug-induced emesis.
Insomnia and Anxiety (Off-Label)
Sedative first-generation agents like Diphenhydramine or Hydroxyzine can help short-term insomnia. Hydroxyzine also occasionally addresses generalized anxiety symptoms, given its calming effect. However, tolerance to sedative properties can develop, diminishing long-term utility.
Anaphylaxis Adjunct
Although epinephrine remains the cornerstone for anaphylaxis, H1 antihistamines (and sometimes H2) can help alleviate cutaneous and mucosal symptoms. They do not replace epinephrine but may shorten the duration of hives or itching.
Adverse Effects and Precautions of H1 Antihistamines
Sedation and CNS Depression
Mainly with first-generation. Driving impairment, slowed reaction times, confusion (especially in the elderly) are all concerns. Simultaneous use with alcohol or other CNS depressants can amplify sedation, raising safety risks.
Anticholinergic Effects
Manifest as dry mouth, blurred vision, urinary retention, constipation, tachycardia. Concerning in older patients at risk for cognitive decline, confusion, or delirium. Prostate enlargement and narrow-angle glaucoma are relative contraindications.
Cardiac Toxicity
Some older second-generation antihistamines were associated with QT prolongation (e.g., Terfenadine, Astemizole), leading to torsades de pointes under certain metabolic inhibition conditions. Modern second-generation agents (e.g., Fexofenadine, Cetirizine, Loratadine) generally lack clinically significant cardiotoxicity when used as directed.
Drug Interactions
Potent cytochrome P450 inhibitors (e.g., ketoconazole, macrolide antibiotics) historically resulted in elevated plasma levels of older second-generation agents, risking cardiotoxicity. While newer agents are safer, checking for interactions remains prudent.
H2 Receptor Antagonists
Mechanism and Examples
Cimetidine, Ranitidine, Famotidine, Nizatidine block H2 receptors on parietal cells, reducing basal and stimulated gastric acid secretion. They were once mainstays for peptic ulcers, GERD, and stress ulcer prophylaxis.
Clinical Roles and Limitations
Though overshadowed by proton pump inhibitors, H2 blockers still treat mild to moderate GERD, especially for on-demand nocturnal acid control. They have a more immediate onset than PPIs but less potent acid suppression. Some commonly used in over-the-counter forms for heartburn relief.
Adverse Effects
Generally well-tolerated. Cimetidine can inhibit P450 enzymes, raising levels of drugs like warfarin, theophylline, or phenytoin. Rarely, it may cause gynecomastia or sexual dysfunction. Famotidine and Ranitidine are less prone to these effects, though the latter faced recall issues for potential impurities in certain preparations.
Emerging Therapies: H3 and H4 Modulators
H3 Receptor Ligands
Preclinical data suggest H3 antagonists could enhance wakefulness, attention, and possibly help in narcolepsy. Some compounds are under clinical trials for cognitive disorders or ADHD. The concept is that blocking inhibitory autoreceptors on histaminergic neurons elevates histamine release, potentially improving vigilance.
H4 Receptor Antagonists
H4’s immunomodulatory function—particularly in pruritus, inflammation, and mast cell chemotaxis—makes it an intriguing target. Some H4 antagonists are explored for allergic asthma, atopic dermatitis, or pruritic conditions. While early-phase trials show promise, broader clinical usage requires more evidence.
Clinical Considerations and Best Practices
Selecting an H1 Antihistamine
For routine allergic rhinitis or urticaria, second-generation non-sedating agents (e.g., Fexofenadine, Loratadine, Cetirizine) are preferred. Older, sedating ones (e.g., Diphenhydramine, Chlorpheniramine) may be chosen if sedation is beneficial (nighttime itching relief) or cost is an issue.
Up-Dosing in Chronic Urticaria
Guidelines often allow up to four times normal daily dose of second-generation antihistamines for refractory chronic urticaria. This approach can improve hives or pruritus control while maintaining a relatively safe profile.
Patient Education
Emphasizing consistent dosing for prophylaxis (e.g., daily administration during allergy season) can optimize symptom control. Patients should understand sedation risk with first-generation agents, avoiding hazardous activities. Checking for potential drug interactions or comorbidities (e.g., BPH, glaucoma) is crucial.
Combination Therapy
For more severe rhinitis, combining a second-generation H1 antihistamine with a nasal corticosteroid or decongestant can be more impactful than monotherapy. Decongestants reduce nasal congestion but can raise blood pressure or heart rate, requiring caution in hypertensive patients.
Role in Anaphylaxis
While epinephrine is definitive, supplementary H1 (and sometimes H2) blockade helps with pruritus, hives, or GI symptoms. Early administration of epinephrine should never be delayed or replaced solely by antihistamines.
Future Directions
Novel Formulations
Sustained-release or prolonged-acting second-generation antihistamine formulations may offer more stable plasma concentrations and extended coverage. There is ongoing interest in intranasal or ocular-specific delivery systems to target local allergic pathways directly.
Personalized Medicine
Variations in histamine receptor genetics or drug metabolism might guide personalized choices. Some individuals experience sedation even with nominally non-sedating agents (like Cetirizine). Pharmacogenomics might eventually refine these selections.
Dual-Target or Combined Agents
In some allergic or inflammatory conditions, combining antihistamine effects with leukotriene antagonism, mast cell stabilization, or anti-platelet activating factor actions might yield synergy. Future drug development may address multiple allergic mediators simultaneously, potentially reducing polypharmacy.
H4 Antagonism in Clinic
If trials confirm beneficial immunoregulatory effects of H4 blockade without significant off-target impacts, novel therapies for atopic dermatitis or chronic itching could emerge. This could revolutionize management of intractable pruritic disorders.
Conclusion
Histamine emerges as a multifaceted mediator crucial for numerous physiological and pathological processes—from allergic inflammation and vascular responses to gastric acid regulation and neurotransmission. Its significance is reflected in the broad utility of antihistamines, which remain foundational for allergy management, pruritus relief, and certain aspects of gastrointestinal disease. The robust distinction between first-generation H1 antagonists (notable sedation and anticholinergic effects) and second-generation agents (reduced CNS penetration, longer action, less sedation) underpins modern clinical practice in allergic rhinitis and urticaria. H2 antagonists further highlight histamine’s importance in controlling gastric acid secretion.
Looking ahead, the continued elucidation of H3 and H4 receptors opens pathways for innovative treatments targeting cognition, wakefulness, pruritus, and immunomodulation. Coupled with advanced formulations and potential combination therapies, the future of histamine-targeted therapies holds promise for patients with allergic, inflammatory, and CNS disorders. By harnessing the knowledge of histamine’s receptor pharmacodynamics and refining antihistamine design, clinicians can deliver safer, more effective symptomatic relief and ultimately enhance quality of life for those affected by histamine-driven conditions.
References
- Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 13th Edition
- Katzung BG, Basic & Clinical Pharmacology, 15th Edition
- Rang HP, Dale MM, Rang & Dale’s Pharmacology, 8th Edition









