Introduction
Fibrinolytics—also known as thrombolytics—are a specialized class of pharmacological agents that dissolve blood clots (thrombi) by catalyzing the conversion of plasminogen to plasmin, the main fibrinolytic enzyme. These drugs are crucial in the acute management of life-threatening conditions such as acute myocardial infarction (AMI), acute ischemic stroke, and massive pulmonary embolism. By reinstating blood flow rapidly, fibrinolytics reduce tissue ischemia and infarction risk. At the same time, because of their substantial capacity to break down thrombi, they can trigger major or sometimes catastrophic bleeding. Thus, clinicians must balance their potent therapeutic benefits against significant hemorrhagic risks.
This comprehensive review examines the pharmacology of fibrinolytics, referencing standard pharmacology textbooks (Goodman & Gilman’s, Katzung, and Rang & Dale’s). It discusses the physiological basis for fibrinolysis, the mechanisms of existing fibrinolytic agents, their pharmacokinetic profiles, clinical indications, adverse effects, and ongoing research aimed at refining current strategies. By understanding how these agents function and the nuances of their usage, clinicians can optimize outcomes for patients requiring urgent thrombolytic therapy.
Physiology of Fibrinolysis
The Hemostatic and Fibrinolytic Balance
Hemostasis involves forming platelet plugs and generating fibrin to prevent excessive blood loss. However, once a damaged vessel stabilizes, fibrinolysis becomes critical in limiting clot extension and facilitating the eventual breakdown of the fibrin plug. This process is orchestrated mainly by plasmin, which digests the fibrin network into fibrin degradation products.
Role of Plasminogen and Plasmin
Plasminogen—the inactive precursor of plasmin—is produced in the liver and circulates in the bloodstream. Upon encountering fibrin surfaces or encountering specific activators (e.g., tissue plasminogen activator, tPA), plasminogen converts into plasmin, which degrades fibrin. The physiological modulators of plasminogen activation include:
- Tissue plasminogen activator (tPA): Endogenously released by endothelial cells.
- Urokinase-type plasminogen activator (uPA): Generated in multiple tissues, playing roles in local fibrinolysis and tissue remodeling.
Regulation of Fibrinolysis
While tPA and uPA form the main drivers of plasminogen conversion, inhibitors such as plasminogen activator inhibitor-1 (PAI-1) and alpha-2 antiplasmin keep fibrinolysis in check, preventing excessive or systemic clot dissolution.
Classification of Fibrinolytic Agents
1. Tissue Plasminogen Activator (tPA) Derivatives
- Alteplase (recombinant tPA)
- Reteplase
- Tenecteplase
2. Non-Specific Agents
- Streptokinase
- Urokinase
3. Other Investigational or Less Commonly Used Agents
- Anistreplase (APSAC)
- Desmoteplase (from vampire bat saliva, under investigation)
Each agent differs in terms of fibrin specificity, half-life, route of administration, antigenicity, and cost. While older agents like streptokinase are cost-effective, they have lower fibrin specificity and higher immunogenic potential. Modern tPA derivatives (e.g., alteplase, tenecteplase) were engineered to prolong half-life or enhance fibrin specificity, potentially improving efficacy and safety.
Mechanisms of Action
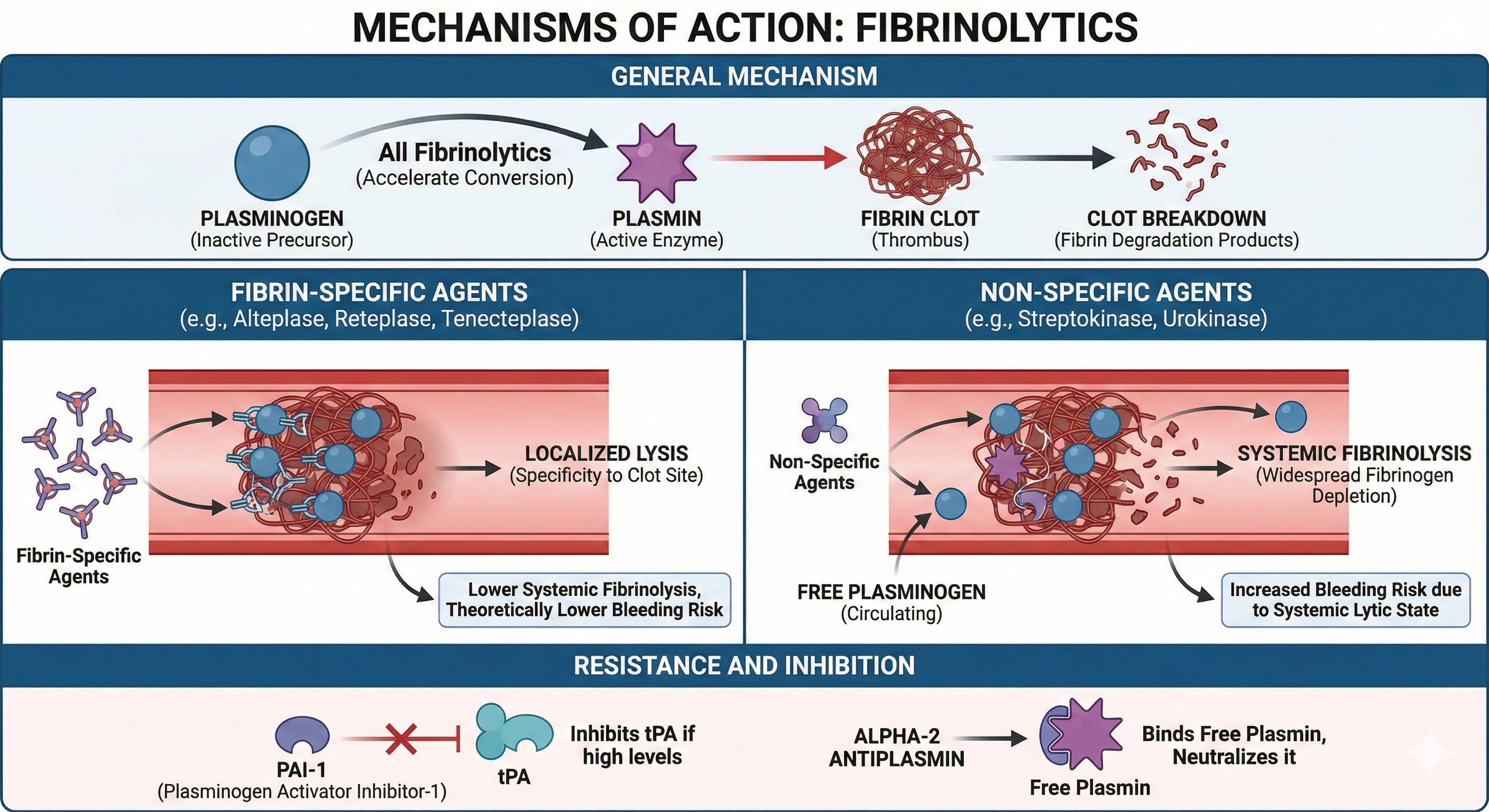
General Mechanism
All fibrinolytics accelerate the conversion of plasminogen to plasmin, leading to the breakdown of fibrin clots. However, fibrinolytics differ in how selectively they localize to fibrin-bound plasminogen or how extensively they affect circulating plasminogen.
Fibrin-Specific vs. Non-Specific Activation
- Fibrin-Specific Agents (Alteplase, Reteplase, Tenecteplase): Have higher affinity for plasminogen bound to fibrin within the thrombus, thus conferring some specificity to the clot site and theoretically lowering systemic fibrinolysis.
- Non-Specific Agents (Streptokinase, Urokinase): Activate free (circulating) plasminogen broadly, potentially causing widespread fibrinogen depletion and systemic lytic states, thereby increasing bleeding risk.
Resistance and Inhibition
- PAI-1 can inhibit tPA if present in high levels.
- Alpha-2 antiplasmin binds free plasmin in circulation, neutralizing it before it can degrade fibrin.
Pharmacokinetics of Key Fibrinolytics
Alteplase (Recombinant tPA)
- Structure: Bioengineered form of natural tPA (527 amino acids).
- Mechanism: Prefers fibrin-bound plasminogen, leading to clot-specific lysis.
- Half-Life: Very short (~5 minutes). Continuous IV infusion is usually required.
- Metabolism: Primarily by hepatic clearance; short terminal half-life ~30–45 minutes.
Reteplase
- Modified tPA lacking certain domains, making it smaller and less glycosylated than alteplase.
- Pharmacokinetics: Longer half-life (13–16 minutes), allowing bolus administration.
- Fibrin Specificity: Somewhat less than alteplase but more selective than streptokinase.
Tenecteplase
- Genetically engineered tPA variant with three amino acid substitutions.
- Pharmacokinetic Advantage: Longer half-life (~20–24 minutes) enables single or double bolus dosing.
- Greater Fibrin Specificity: More resistant to PAI-1, thus potentially improved efficacy and decreased bleeding risk compared to alteplase.
Streptokinase
- Bacterial Protein: Derived from beta-hemolytic streptococci.
- Mechanism: Forms a complex with plasminogen, triggering broad conversion of plasminogen to plasmin.
- Half-Life: Approximately 30 minutes. Often given by prolonged infusion.
- Immunogenic: Antigenic because of its bacterial origin, can provoke allergic reactions and neutralizing antibodies.
Urokinase
- Human Enzyme produced by renal tubular cells, purified from urine or cultured kidney cells.
- Non-Fibrin-Specific: Directly converts plasminogen to plasmin in plasma.
- Half-Life: ~10–15 minutes. Continuous infusion needed for sustained effect.
- Immunogenicity: Less immunogenic than streptokinase but still can induce systemic fibrinolysis.
Anistreplase (APSAC)
- Complex of Streptokinase + Plasminogen with anisoyl protective group.
- Prodrug: The anisoyl group extends half-life by delaying streptokinase-plasminogen complex activation until the agent reaches clot sites.
- Use: Historically used in acute MI, though overshadowed by newer agents.
Clinical Indications
1. Acute Myocardial Infarction (ST-Elevation MI)
Timely fibrinolysis can salvage myocardium if percutaneous coronary intervention (PCI) is not readily available. The standard fibrinolytics used here include alteplase, reteplase, or tenecteplase. The therapy is most beneficial if given within 12 hours of symptom onset—ideally within the first 1–3 hours.
2. Acute Ischemic Stroke
Alteplase is the main fibrinolytic approved for stroke management, administered within a narrow time window (up to ~3–4.5 hours after stroke onset in eligible patients). This can restore blood flow to the ischemic brain, limiting infarct size.
3. Massive Pulmonary Embolism
In cases of significant hemodynamic instability or shock, fibrinolytic therapy can be life-saving by rapidly dissolving the obstructing clot in pulmonary arteries. Alteplase or streptokinase or other agents may be used.
4. Peripheral Arterial Occlusion / Limb-Threatening Ischemia
Local catheter-directed fibrinolysis can salvage an ischemic limb. Typically administered via infusion directly into the thrombus site.
5. Other Off-Label or Investigational Uses
- Clearance of occluded catheters or hemodialysis access.
- Loculated pleural effusions (in conjunction with drainage).
- Intra-ventricular hemorrhage (low-dose tPA injections in certain neurocritical care protocols; still under study).
Dosing Strategies
Bolus vs. Infusion
- Rapid Bolus: Agents like tenecteplase, reteplase can be administered as one or two bolus injections for STEMI.
- Continuous Infusion: Classic approach with alteplase or streptokinase in MI, stroke, or PE. The infusion duration differs among indications (30–90 minutes for MI, up to 60 minutes or more for stroke).
Weight-Based Dosing
Many regimens (especially alteplase) tailor the dose to the patient’s body weight, capping at certain maxima. For instance, in ischemic stroke, the total alteplase dose is 0.9 mg/kg, with 10% as an initial bolus.
Adjunct Antithrombotic Therapy
- Antiplatelet Agents: Aspirin and, in some settings, P2Y₁₂ inhibitors can be combined for STEMI. However, in acute stroke management, guidelines caution about concomitant antiplatelet usage until after the fibrinolytic infusion.
- Heparin: May be used or withheld depending on guidelines, indication, and the agent selected. For instance, some fibrinolytic regimens for STEMI incorporate intravenous heparin concurrently.
Adverse Effects of Fibrinolytics
Hemorrhagic Complications
The most serious adverse effect is intracranial hemorrhage (ICH), which can be fatal or result in severe neurological disability. Risk factors for bleeding include advanced age, hypertension, recent surgery, or a history of stroke. Thus, thorough screening for contraindications is vital before initiating therapy.
Reperfusion Arrhythmias
In myocardial infarction, abrupt restoration of blood flow may provoke transient ventricular arrhythmias or conduction disturbances. Typically, these are self-limited or managed with antiarrhythmic strategies.
Hypotension and Allergic Reactions
- Streptokinase can trigger hypotension and anaphylactic reactions due to its bacterial origin.
- Urokinase and recombinant tPA derivatives are less immunogenic but still can cause infusion-related reactions in rare cases.
Systemic Lytic State and Fibrinogen Depletion
Non-fibrin-specific agents (streptokinase, urokinase) deplete circulating fibrinogen, increasing bleeding risk for hours to days. Serial fibrinogen levels may guide therapy continuation or transfusion support (e.g., cryoprecipitate if significantly low).
Contraindications and Precautions
Absolute Contraindications
- Active internal bleeding
- History of intracranial hemorrhage or structural cerebral lesion (aneurysm, AV malformation)
- Intracranial or intraspinal surgery within recent months
- Severe uncontrolled hypertension (systolic >185 mmHg or diastolic >110 mmHg that cannot be lowered)
- Suspected aortic dissection
- Recent intracranial or spinal trauma
Relative Contraindications
- Recent major surgery (<2–4 weeks)
- Active peptic ulcer disease
- Pregnancy
- Current use of anticoagulants (INR might complicate risk assessment)
- Advanced age or very low body weight
- Severe hepatic or renal impairment
Guidelines for stroke, MI, and PE present nuanced checklists verifying each patient’s risk-benefit ratio. Relative contraindications may be overridden in life-threatening situations with thorough caution.
Strategies to Mitigate Bleeding Risks
Inclusion-Exclusion Criteria
Strictly adhering to well-defined criteria for time since symptom onset, blood pressure thresholds, and recent surgical or hemorrhagic events lowers hemorrhagic complications.
Blood Pressure Control
Aggressively treating severe hypertension before administering fibrinolytics reduces intracranial hemorrhage risk. Intravenous antihypertensives are used in stroke or STEMI protocols if BP exceeds recommended thresholds.
Avoiding Traumatic Procedures
During fibrinolysis, clinicians should minimize invasive procedures (e.g., intramuscular injections, central venous catheters) that could provoke bleeding. If necessary, such procedures are often performed before starting a thrombolytic or delayed until the agent’s effect wanes.
Monitoring and Early Intervention
Frequent neurological checks in stroke patients or hemodynamic monitoring in PE/MI contexts are critical. The earliest signs of bleeding (e.g., unexpected drop in hematocrit, neurological changes) prompt discontinuation of the fibrinolytic infusion and further diagnostic imaging or supportive care.
Reversal and Management of Fibrinolytic Bleeding
Pharmacological Reversal Agents
- Aminocaproic Acid or Tranexamic Acid: Lysine analogs that competitively inhibit plasminogen binding to fibrin, effectively halting fibrinolysis. These agents can be administered if life-threatening bleeding occurs post-fibrinolytic therapy.
- Cryoprecipitate: Rich in fibrinogen, Factor VIII, and XIII, may restore depleted coagulation factors.
- Platelets, Fresh Frozen Plasma (FFP), or Prothrombin Complex Concentrates (PCC) could be considered if coagulopathy from combined anticoagulants and fibrinolytics is severe.
Supportive Measures
- Temporary discontinuation of all anticoagulants and antiplatelet drugs.
- Intensified vital sign monitoring, imaging (CT scans if suspected intracranial hemorrhage), and surgical consultation (e.g., for compartment syndrome or ongoing hemorrhage requiring intervention).
Efficacy and Outcome Predictors
Time Is Muscle/Brain
Fibrinolytic success correlates inversely with the delay between symptom onset and drug administration. For myocardial infarction, the earlier the reperfusion, the lower the mortality and better left ventricular function preservation. Similarly, with ischemic stroke, timely therapy is crucial to salvage penumbral tissue.
Clinical Scales
- TIMI (Thrombolysis in Myocardial Infarction) Flow Grade or Blush Grade help gauge coronary reperfusion post-fibrinolysis.
- NIH Stroke Scale improvement suggests successful clot lysis in ischemic stroke.
Combination Strategies
Pharmacoinvasive approaches—where fibrinolysis is started early, followed by urgent PCI—can yield favorable outcomes in areas where timely PCI is unavailable. However, careful coordination is needed to mitigate bleeding risk upon subsequent invasive procedures.
Head-to-Head Comparisons
Streptokinase vs. Alteplase
- Streptokinase is cheaper, widely available in resource-limited environments, but less fibrin-specific and more immunogenic.
- Alteplase is costlier but offers improved fibrin specificity, leading to somewhat better patency rates and potentially reduced non-cerebral bleeding—although intracranial hemorrhage rates can be similar when comparing large trials in AMI.
Tenecteplase vs. Alteplase
- Tenecteplase is given as a single bolus, simplifying administration. Some data shows similar or slightly better outcomes and decreased bleeding rates in certain populations.
- Alteplase is still the standard for acute ischemic stroke due to more extensive clinical trial experience and regulatory approval.
Reteplase
- A double-bolus regimen (two bolus injections 30 minutes apart).
- Has intermediate fibrin specificity and simpler dosing than alteplase infusion.
Special Populations
Elderly Patients
An elevated risk of intracranial hemorrhage and comorbidities must be weighed against potential survival or functional recovery benefits. Some guidelines reduce aggressiveness in borderline indications for the elderly, especially if time since symptom onset has extended significantly.
Pediatric Thrombosis
Pediatric arterial or venous thrombosis is rare; fibrinolytics may be selectively considered in catastrophic events (e.g., extensive cerebral venous sinus thrombosis). Dosing protocols are not as standardized, necessitating expert pediatric hematology consultation.
Pregnancy
Generally contraindicated due to miscarriage risk, placental hemorrhage, or postpartum hemorrhage. However, in life-threatening maternal thrombosis (e.g., massive pulmonary embolism), off-label usage may be considered with extreme caution.
Renal or Hepatic Impairment
Renal or hepatic dysfunction complicates the risk of coagulopathy and drug clearance. Some fibrinolytics or adjuvant therapies (like heparin) must be dose-adjusted or used with caution, taking into account bleeding propensity and metabolic elimination pathways.
Future Directions and Emerging Therapies
Improved Fibrin-Targeting
Research seeks next-generation molecules that exploit novel binding sites on fibrin or plasminogen to achieve enhanced clot selectivity, diminishing hemorrhage risk. Variants of tPA more resistant to PAI-1 or with advanced domain modifications point to incremental improvements.
Nanoparticle-Delivered Thrombolytics
Nanomedicine approaches propose encapsulating or binding fibrinolytics to targeted drug carriers for local clot release. By restricting drug exposure to the thrombus region, systemic side effects can be minimized.
Combination with Mechanical Thrombectomy
For acute ischemic stroke, mechanical thrombectomy is now standard in large vessel occlusions. Fibrinolytics can be used adjunctively before or during the procedure to facilitate partial clot dissolution. However, mechanical revascularization has overshadowed fibrinolysis in many stroke centers with advanced endovascular capabilities.
Gene Therapies and Biological Strategies
Investigational directions include genetic modifications to enhance local fibrinolytic potential or recombinant viruses delivering fibrinolytic enzymes directly to the clot. Still in preclinical phases, these strategies await further exploration.
Practical Considerations in Clinical Practice
- Selecting the Agent: Clinicians weigh body weight, time since onset, site of thrombosis, cost, immunogenic concerns, and local protocols.
- Dose Accuracy: Overdosing fibrinolytics escalates hemorrhage risk, while underdosing may yield incomplete reperfusion.
- Monitoring: Vital signs, neurological status, ECG changes, bleeding manifestations, and lab parameters (fibrinogen, hemoglobin) guide ongoing therapy adjustments.
- Informed Consent: Communicating both the risk of hemorrhagic stroke and potential functional benefit is essential in emergent scenarios if feasible.
- Institutional Protocols: Many hospitals have standardized checklists verifying indications, contraindications, and safe administration procedures for stroke or STEMI.
- Post-Thrombolysis Care: Ongoing supportive therapy, bridging to other anticoagulants (if indicated), controlling comorbidities (hypertension, diabetes), and rehabilitative measures are integral to optimizing outcomes.
Conclusion
Fibrinolytics constitute a critical therapeutic strategy for acute thrombotic emergencies—chiefly acute myocardial infarction, ischemic stroke, and massive pulmonary embolism—when prompt restoration of perfusion can prevent death or severe organ damage. They achieve their effects by catalyzing plasmin-mediated fibrin degradation, effectively dissolving clots. Over recent decades, advances in recombinant DNA technology have yielded fibrin-specific agents (e.g., alteplase, tenecteplase) that afford superior reperfusion rates with somewhat reduced systemic fibrinolysis compared to older drugs (e.g., streptokinase). Even so, intracranial hemorrhage and other severe bleeding complications remain formidable hazards and define the narrow risk-benefit window in which fibrinolysis is deemed acceptable.
Rational use mandates strict adherence to guidelines, careful patient selection, and meticulous management of potential bleeding. Adjunct therapies—antiplatelet agents, anticoagulants, or mechanical interventions—are individualized depending on the clinical scenario, whether it be myocardial infarction, cerebral infarction, or massive pulmonary embolism. On the horizon, refinements in molecular engineering, nanotechnology-based approaches, and synergy with endovascular treatments promise further improvements in the safety and efficacy profile of fibrinolytic therapy. By staying abreast of these developments and maintaining vigilant risk assessment, clinicians can deliver targeted, life-saving interventions for acute thrombotic events while minimizing harm.
References
- Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 13th Edition
- Katzung BG, Basic & Clinical Pharmacology, 15th Edition
- Rang HP, Dale MM, Rang & Dale’s Pharmacology, 8th Edition
Medical Disclaimer
The medical information on this post is for general educational purposes only and is provided by Pharmacology Mentor. While we strive to keep content current and accurate, Pharmacology Mentor makes no representations or warranties, express or implied, regarding the completeness, accuracy, reliability, suitability, or availability of the post, the website, or any information, products, services, or related graphics for any purpose. This content is not a substitute for professional medical advice, diagnosis, or treatment; always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition and never disregard or delay seeking professional advice because of something you have read here. Reliance on any information provided is solely at your own risk.