INTRODUCTION
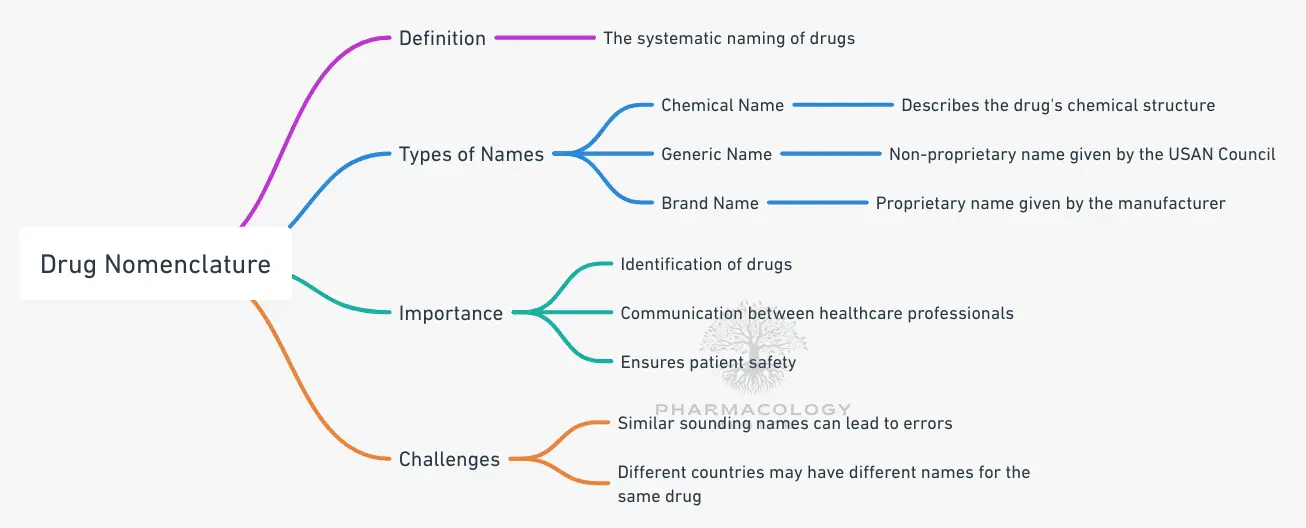
Drug nomenclature is a critical and highly specialized area within pharmaceutical science, involving the systematic naming of medicinal substances for rigorous clarity in healthcare settings, research environments, and regulatory frameworks Accurate drug naming is integral to communication among healthcare professionals, researchers, and patients. Without strict, standardized nomenclature protocols, the risk of medication errors and confusion in prescription or research settings increases significantly. Naming a drug accurately ensures clear identification across international borders, buttresses pharmacovigilance efforts, and establishes the most efficient path for collaboration among scientists, healthcare providers, and regulatory organizations.
The process of deciding what a drug should be called is anything but simple, entailing scientific, regulatory, and sometimes even commercial considerations. Factors such as chemical composition, clinical effects, mechanistic pathways, legal requirements, marketing, and brand identity can all shape a drug’s name. When discussing drug nomenclature, it is useful to remember that it is not only about labeling a compound but also about ensuring patient safety and promoting global consistency in medical practice. Healthcare professionals rely on drug names that are unambiguous, standardized, and easily recognizable. Moreover, scientific evolution continually reshapes the landscape, introducing novel molecules and advancing therapeutic modalities such as biologics, biosimilars, gene therapies, and targeted treatments. This evolution leads to the continuous refinement and revision of naming strategies so that nomenclature remains relevant, accurate, and reflective of the underlying science.
The modern field of drug nomenclature involves more than just a categorization of substances; it relies on contributions from the World Health Organization (WHO), national and international regulatory agencies, professional associations, linguists, patent lawyers, marketing teams, and specialized drug nomenclature committees. These bodies oversee the creation and standardization of drug names at different stages in a drug’s life cycle, from development to commercial distribution.
In this chapter, we will delve deep into the systematically organized naming conventions for drugs and examine how each name type—chemical, generic, and brand—plays a key role in pharmaceutical practice worldwide. We will also explore the role played by the WHO’s International Nonproprietary Names (INN) program the interplay between national naming agencies, and the importance of these conventions in fostering patient safety, clarifying medical documentation, and even influencing commercial interests. Finally, we will consider how drug nomenclature is evolving in response to the surge of large-molecule biologics, gene therapies, and other cutting-edge treatments, providing a keen insight into the future of naming strategies in this rapidly changing domain.
THE BASIC ELEMENTS OF DRUG NOMENCLATURE
At first glance, it may appear that a single drug has countless names. Indeed, drug nomenclature often involves at least three major categories: the chemical name, the generic (nonproprietary) name, and the brand (or proprietary) name.
Each name serves a distinct purpose:
- Chemical (IUPAC) Name:
• Conveys the precise organic or inorganic chemical structure.
• Follows systematic rules set by the International Union of Pure and Applied Chemistry (IUPAC).
• Generally too long and complex for routine clinical usage. - Generic (Nonproprietary) Name:
• Assigned by official bodies such as the U.S. Adopted Name (USAN) Council in the United States or approved by the WHO under the INN program
• Unowned by any company, promoting consistency and safety.
• Recognizable in many countries and used commonly in prescriptions. - Brand (Proprietary) Name:
• Owned by the manufacturer or patent-holder.
• Often designed for marketing and brand identity.
• Managed by regulatory bodies to prevent confusion with other existing names.
While these three categories of drug names support different functions, major overlaps occur in regulatory practice, intellectual property considerations, and real-world clinical usage. For instance, a new drug may be known by a coded identifier during research, carry a complex chemical descriptor within its laboratory documentation, and be submitted to WHO or a national body for a proposed generic name. It will, in all likelihood, also acquire a commercially appealing name once marketing authorization is sought. Managing these naming layers effectively is crucial for clear communication and to maintain safety across international markets.
THE CHEMICAL NAME
A drug’s chemical name is a reflection of its molecular structure. This name is governed by the internationally recognized nomenclature guidelines published by IUPAC. A chemical name explicitly describes the arrangement and type of atoms within the molecule, making it the most comprehensive depiction of the drug’s fundamental chemistry. Nevertheless, chemical names often become unwieldy in everyday healthcare and business scenarios due to their length and complexity. For instance, consider a hypothetical compound containing multiple functional groups and stereocenters; its IUPAC name can easily stretch into dozens of syllables or more.
Chemists and researchers rely on this precise chemistry-based nomenclature to ensure they are always discussing the exact same molecule, especially when verifying chemical purity, analyzing potential analogues, and comparing structures. Detailed knowledge of a drug’s molecular composition is frequently necessary for drug discovery, quality control, and the early stages of pharmaceutical research.
Furthermore, the chemical name matters when patent offices and regulatory agencies assess novel substances for intellectual property rights or for scientific documentation. Any ambiguity in the chemical name risks confusion between similarly structured molecules that may have noticeably different pharmacological activities or safety profiles.
Unlike the more succinct generic and brand names, a drug’s chemical name is rarely used by prescribers or even by most pharmacists in day-to-day settings. Its primary function is to describe the structure unambiguously in specialized documentation. This ensures minimal confusion for scientists working in drug design and offers clarity in the scientific literature, where elaborate chemical discussions require exact structural representation. Nevertheless, when a new compound is first concocted in a lab, that initial IUPAC-based identifier is often the only formal name the drug candidate has until it progresses in the developmental and regulatory pipeline.
Certain preliminary or investigational designations may also be associated with a compound, often referred to as “laboratory codes” or “internal codes” used by pharmaceutical companies. However, these in-house codes do not usually become official nomenclatures or universal designators. In summary, the chemical name remains indispensable in the scientific process and acts as the bedrock on which a drug’s subsequent naming layers are built.
THE GENERIC (NONPROPRIETARY) NAME
Once a drug shows promise and moves beyond the preliminary investigative stages, the drug sponsor applies to relevant authorities for a generic name. Known as the nonproprietary name, this moniker is recognized in most regions around the world, providing a shared reference by which all stakeholders can identify the substance. As the name suggests, a generic name is not owned by any single entity and is intended for widespread usage, unencumbered by trademark or patent restrictions . This approach fosters clarity, standardization, and public health benefits, since healthcare professionals worldwide can reference an easily recognizable name.
In the United States, the United States Adopted Names (USAN) Council is responsible for assigning generic names to drugs. The WHO, through its INN program, approves these nonproprietary names to promote global uniformity, serving as a critical reference point even for countries with their own naming agencies. Each new proposed generic name is carefully evaluated based on established guidelines that consider linguistic simplicity, minimal potential for confusion with existing product names, and reflection of the drug’s pharmacological class names often incorporate a “stem” that indicates the drug’s class or mechanism of action. For instance, many beta-blockers end with “-olol,” while certain angiotensin-converting enzyme (ACE) inhibitors end with “-pril.” This approach helps healthcare providers quickly identify a drug’s general mechanism or therapeutic class, contributing greatly to safe prescribing practices. Over time, these stems evolve in tandem with advances in pharmacology, requiring ongoing updates to reflect newly discovered mechanisms or drug classes.
Globally, the adoption of a uniform nonproprietary name reduces confusion. Even if a single drug is sold under multiple brand names in various countries, the generic name ensures that prescribers, pharmacists, and researchers can always identify the agent. This uniformity is especially valuable for pharmacovigilance, the process of monitoring the effects of medical drugs after they have been licensed for use. When a safety alert is raised regarding a specific substance, the generic name ensures that health agencies, hospitals, and prescribers can coordinate effectively across borders, referencing a common nomenclature even if multiple brand names exist in different markets.
Notably, the generic name also factors into public health decisions and policies like drug procurement, essential medicines listings, and insurance reimbursement frameworks. Since nonproprietary names do not belong to a single company, generics advertising, healthcare guidelines, and coverage policies frequently rely on generic nomenclature. This approach emphasizes competition and cost savings in pharmaceutical markets, offering equitable access to medications. Indeed, the generic name has become synonymous with open availability, cost-effective prescription, and broad-based international recognition.
THE BRAND (PROPRIETARY) NAME
While the chemical and generic names primarily serve clinical and regulatory clarity, the brand name—or proprietary name—is motivated largely by intellectual property protection and marketing considerations. The manufacturer or patent-holder typically selects the brand name and registers it as a trademark. Regulatory entities, such as the Food and Drug Administration (FDA) in the United States, must approve this name to prevent it from sounding too similar to existing drug names and thus minimize confusion or medical errors.
Brand names often aim for memorability, market positioning, and consumer appeal. Subtle references to a drug’s effect, intended use, or brand identity might be embedded in the name. However, the trademark cannot be too suggestive of the drug’s specific uses or benefits, due to regulatory restrictions meant to avoid misleading claims. The brand name can be an enormous asset to pharmaceutical companies, particularly if the drug gains a strong reputation or garners extensive market share. Patent protection and associated marketing campaigns create distinctive brand equity. Even after the patent expires and cheaper generic versions of the drug become available, the proprietary name often holds sway in the marketplace among clinicians and patients who may have first encountered the drug in its brand form.
An example of the tension between brand recognition and universal acceptance is the classic acetaminophen vs. paracetamol naming convention. In the United States, acetaminophen (the generic name) is widely recognized under the proprietary brand Tylenol®, whereas globally, paracetamol is more commonly acknowledged. This kind of regional variation can lead to confusion if clinicians from different countries are not aware that these names refer to the same active ingredient. However, the universal generic name (“acetaminophen”) and the internationally recognized nonproprietary name (“paracetamol”) function precisely to ensure clarity and safety, despite the existence of a famous brand name.
It is essential that clinicians, scientists, and regulators keep track of the official brand names in use, especially in countries where multiple brand names for the same drug may compete in the marketplace. Brand names, through promotional activities and manufacturer-driven campaigns, are most visible to the public, often overshadowing the generic name in common parlance. Nevertheless, nonproprietary names remain fundamental references for safe prescribing and dispensing, while proprietary names frequently serve to strengthen corporate identity and patient recognition.
ROLE OF WHO AND INTERNATIONAL NONPROPRIETARY NAMES
Central to the standardization of drug nomenclature is the WHO’s International Nonproprietary Names (INN) program. This initiative confers official, universally recognized names for pharmaceutical substances, ensuring that healthcare professionals, pharmacists, and patients worldwide can identify a specific drug using a consistent name.
The INN program is built around a review process in which drug developers propose a name, followed by extensive evaluation by national nomenclature committees, professional societies, and the WHO’s INN experts. The final approved name is then published, entered into the global record, and used for the remainder of the drug’s lifespan.
One of the most notable features of the INN system is the use of common stems in certain therapeutically relevant groups. For instance, monoclonal antibodies frequently include “-mab” as part of their nonproprietary name, clearly indicating their nature to professionals reviewing prescribing information. This consistent approach simplifies classification, prescription writing, educational programs, and research. It also facilitates seamless scientific communication when comparing therapies across different countries.
Obtaining an INN is not just a formality; it is often a prerequisite for global drug distribution and plays a pivotal role in the regulatory review process in many jurisdictions. Health agencies, including the European Medicines Agency (EMA) and the FDA, rely on the INN program’s naming conventions to ensure standardized nomenclature in submission documents, product labels, and promotional materials. This approach vastly cuts down on the potential for confusion creating public health risks.
For drug developers and manufacturers, aligning branding activities with the assigned INN is a strategic imperative. While the INN is nonproprietary, sponsors often attempt to devise brand names that reference or play upon the generic name so that healthcare professionals equate the drug’s brand with its scientific or clinical category. Still, the INN itself cannot be trademarked, preserving the principle of open, global usage.
The WHO’s program is particularly beneficial for low- and middle-income countries lacking extensive local nomenclature resources. By adopting the globally recognized INN, these healthcare systems increase prescribing accuracy, build robust procurement strategies, and ensure that the same active substances are recognized and used safely wherever they are needed. As pharmaceuticals become more complex and more countries participate in global commerce, the importance of a unifying naming system becomes even more evident.
THE EVOLVING LANDSCAPE: MACROMOLECULES, BIOLOGICS, AND ADVANCED THERAPIES
The rise in development of biologics, biosimilars, and advanced therapies such as cell and gene therapies has led to new complexities in drug nomenclature. Traditionally, small-molecule therapies dominated the market, and the existing nomenclature guidelines were well-equipped to handle them. However, large, protein-based molecules (e.g., monoclonal antibodies), RNA-based treatments, and various cell-engineered products are inherently more complex, often requiring new or adapted naming conventions.
Monoclonal antibodies—a rapidly expanding category—employ the “-mab” stem in their nonproprietary names However, sub-classifications offer additional layers of meaning. For instance, the presence of “-umab,” “-zumab,” “-ximab,” or “-amab” in the name can indicate if the monoclonal antibody is human, humanized, chimeric, or has other specific derivations. Similarly, new nomenclature guidelines have emerged for gene therapies, which are designed to replace or modify genetic information. These therapies often incorporate complex vectors, transgenes, and other molecular constructs far removed from traditional small-molecule pharmaceuticals.
Biosimilars add a further level of complexity. By definition, a biosimilar is highly similar to an already approved biologic, but subtle differences—due to manufacturing processes—mean these agents are not exact replicas. Nomenclature committees face the challenge of distinguishing biosimilars from their reference products while acknowledging their close relationship. Regulatory bodies such as the FDA and EMA have introduced suffix requirements or other distinguishing naming conventions to facilitate pharmacovigilance.
In parallel, advanced therapies like CAR T-cells (chimeric antigen receptor T-cells) combine genetics and immunology to engineer patients’ cells ex vivo before reinfusion. The naming for these cell-based products must capture the unique characteristics of the therapeutic cell line, vector, or genetic modification. Consequently, nomenclature in advanced therapy is less about single molecules and more about describing a complex, living, or semi-living product.
Given the rapidly advancing technologies underlying these therapies, nomenclature protocols are in a state of flux. The need for precise yet practicable naming strategies is critical. Regulatory authorities often collaborate with groups like the WHO and professional associations, ensuring alignment in how new classes of drugs (and “drugs” that are, in fact, living therapeutics) are named. The goal remains to minimize clinical confusion while reinforcing the known relationships between reference products, biosimilars, and novel therapies.
THE FUTURE OF DRUG NOMENCLATURE
Drug nomenclature will likely continue to evolve, fueled by the increasing complexity of therapeutic agents, globalization, and the need for regulatory harmonization. Traditional rules and patterns developed for small molecules may not always translate well to large protein complexes, peptide-based therapies, gene editing constructs, or living cell products. Consequently, we can expect further dialogue between stakeholders—industry, scientists, lawmakers, health agencies, and professional societies—to ensure that nomenclature frameworks remain fit for purpose.
One of the primary challenges is to strike a balance between clarity for healthcare professionals and comprehensibility for patients. As patients become more active participants in medical decision-making, having drug names that are comprehensible and less intimidating can be beneficial. In parallel, patients who engage in self-directed research and consult numerous sources need to be able to differentiate easily between brand and generic names. Simpler but unambiguous naming systems could also contribute to improved adherence and reduced medication errors.
Meanwhile, the overall push for cost-effective healthcare underscores the importance of standardized names in enabling quicker generics entry and facilitating price competition. Without a streamlined approach that fosters the generation and adoption of generic names, confusion between various brand names can stifle competition and limit patient access to low-cost alternatives.
Finally, advanced technologies and data analytics tools may develop into integral components of nomenclature practice. For instance, large-scale textual data mining and AI-driven analysis of nomenclature usage in real-time prescriptions, pharmacy records, and academic literature may enable faster identification of confusing or overlapping names. This valuable information could guide future naming guidelines and help refine existing systems to maintain clarity in an ever-evolving therapeutic landscape.
CONCLUSION
Drug nomenclature stands at the intersection of science, regulation, linguistics, intellectual property, and public health. Although at times overshadowed by drug development breakthroughs, naming is a critical link in the healthcare chain. From the chemically detailed IUPAC designation that ensures molecular precision, to the WHO-assigned INN that serves the global community, to the brand names that become household words—every step of drug nomenclature exists in service of clarity, safety, and accessibility in modern medicine.
As new therapies bring hope to patients with complex diseases, nomenclature will continue to play an indispensable role in ensuring that these treatments are properly identified, monitored, and regulated worldwide. By maintaining a harmonized approach, rooted in established scientific and regulatory principles while flexible enough to accommodate emerging technologies, the field of drug nomenclature will remain a cornerstone of safer, more effective global healthcare. Its significance will only grow as treatments become more sophisticated, collaborations more global, and the need for unambiguous drug identification more pressing than ever before.
Through rigorous processes and cooperative efforts across nations and scientific disciplines, drug nomenclature has evolved into a robust system capable of withstanding the challenges of a rapidly changing pharmaceutical landscape. Underpinning it all is the ethos that naming is not merely a formality, but a foundational aspect of patient safety, therapeutic clarity, and progress in drug research and development.
Medical Disclaimer
The medical information on this post is for general educational purposes only and is provided by Pharmacology Mentor. While we strive to keep content current and accurate, Pharmacology Mentor makes no representations or warranties, express or implied, regarding the completeness, accuracy, reliability, suitability, or availability of the post, the website, or any information, products, services, or related graphics for any purpose. This content is not a substitute for professional medical advice, diagnosis, or treatment; always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition and never disregard or delay seeking professional advice because of something you have read here. Reliance on any information provided is solely at your own risk.