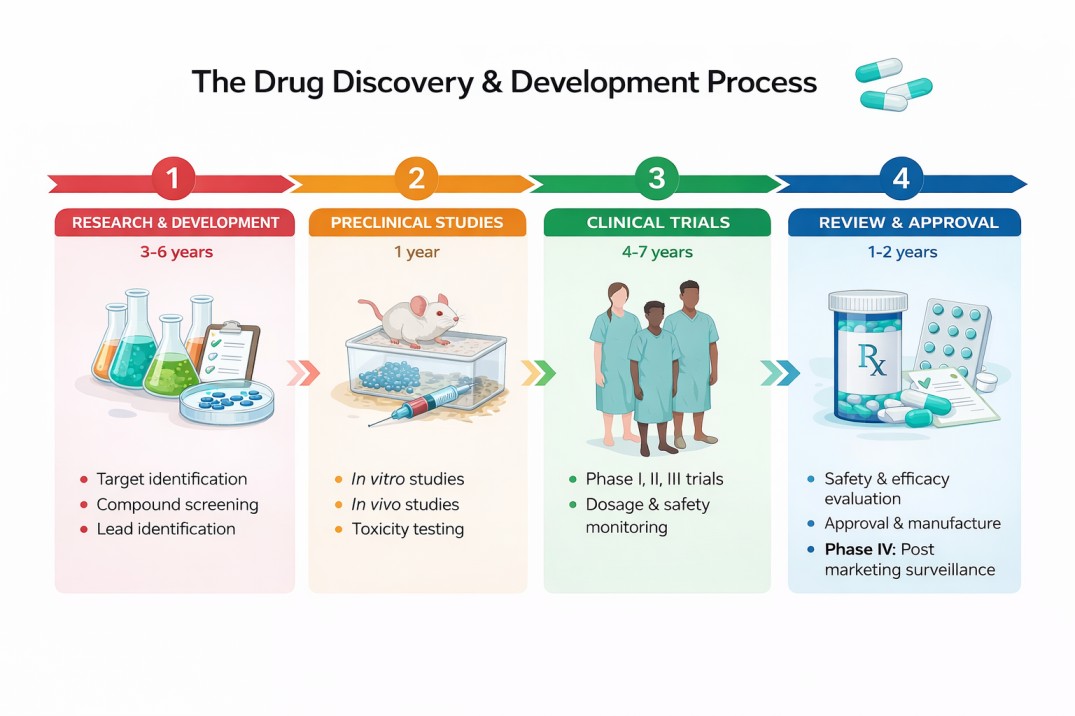
Drug discovery and development is an intricate and multifaceted process that encompasses identifying, designing, and testing prospective new drugs. The primary objective is to discover secure and effective treatments for various ailments and conditions. This composition will delve into the various stages of drug design and screening and the several phases of clinical trials.
Drug Design and Screening
In the initial phases of drug discovery, researchers identify potential drug candidates through various techniques. Rational drug design and high-throughput screening are two of the most prevalent methods.
Rational Drug Design
Rational drug design is an approach in which scientists employ their knowledge of a target protein’s molecular structure and function to devise a molecule that interacts with it in a specific manner. This approach frequently entails computer-aided drug design (CADD) tools to simulate and forecast the interactions between the drug candidate and the target protein. Rational drug design has been effective in producing novel treatments for a variety of diseases, including HIV and cancer.
High-Throughput Screening
High-throughput screening (HTS) is another approach utilized in drug discovery. This technique entails assessing a large number of compounds for their potential to interact with a target protein or other biological targets. Automated equipment rapidly screens thousands to millions of compounds, allowing researchers to identify potential drug candidates quickly. The compounds that show promising activity in these initial screens are subsequently studied and optimized.
Structure-Activity Relationships (SAR)
Researchers identify potential drug candidates and investigate their structure-activity relationships (SAR). This entails analyzing how a compound’s chemical structure impacts its biological activity. By comprehending SAR, researchers can alter the compound’s structure to enhance its effectiveness, reduce side effects, or increase its ability to reach the target site in the body.
After the initial discovery and identification of a promising compound, the next crucial step in drug development is preclinical trials. These trials are conducted before any human testing takes place and serve as a rigorous assessment to determine whether the drug is safe and effective enough to proceed to human trials.
Preclinical Trials
Preclinical trials typically involve both in vitro (test tube or cell culture) and in vivo (animal) studies. Researchers focus on understanding the drug’s mechanism of action, its pharmacokinetics (how it moves through the body), pharmacodynamics (its effects on the body), and toxicology (potential harmful effects). These studies aim to answer several key questions:
- Does the drug have the intended effect at the cellular or molecular level?
- What are the pharmacokinetic properties of the drug, such as absorption, distribution, metabolism, and excretion?
- What are the pharmacodynamic properties, including the physiological effects and potential therapeutic benefits?
- Is the drug safe? What are the toxic effects, and what is the range of safe dosages?
- Does the drug interact with other medications?
- Can the drug be formulated into a form suitable for human administration, such as a pill or injection?
If a drug successfully passes the preclinical stage, the data is compiled and submitted to regulatory agencies like the U.S. Food and Drug Administration (FDA) or the European Medicines Agency (EMA) for approval to move on to Phase 1 clinical trials. These are the first trials involving human participants and primarily focus on safety and dosage.
It’s worth noting that many compounds fail during the preclinical stage due to a lack of efficacy, unacceptable toxicity levels, or other issues. Those that do pass this stage have cleared a significant hurdle but still have a long journey ahead, including multiple phases of clinical trials, before they can be approved for general use.
Thus, preclinical trials serve as a critical filter in the drug development pipeline, allowing only the most promising candidates to proceed to human testing.
Clinical Trials
After a drug successfully navigates the preclinical trial stage, demonstrating promising efficacy, acceptable safety profiles, and favourable pharmacokinetic and pharmacodynamic properties, it moves on to the clinical trial phase. This transition marks a significant milestone: The drug is now ready to be tested in humans for the first time.
Phase I: Safety and Dosage
The primary goal of Phase I clinical trials is to evaluate the safety and tolerance of a new drug in a small group of healthy volunteers. Researchers also use this phase to determine the appropriate dosage of the drug. They closely monitor participants for any adverse reactions and adjust the dosage as needed to find the most effective and safe dose.
- Objective: To gather preliminary data on pharmacodynamics and pharmacokinetics.
- Participants: Usually 10-15.
- Duration: A few weeks to a couple of months.
Phase II: Efficacy and Side Effects
Phase II clinical trials involve a larger group of participants, typically including patients with the condition the drug is intended to treat. In this phase, researchers assess the drug’s efficacy in treating the condition as well as any side effects it may cause. This phase aids in determining whether the drug has therapeutic potential and is safe enough to proceed to the next stage of testing.
- Objective: To determine safety and dosage parameters.
- Participants: 20-100 healthy volunteers or patients.
- Duration: Several months to a year.
Phase III: Efficacy, Monitoring, and Comparison
In Phase III clinical trials, the drug is tested on an even larger group of patients to confirm its efficacy, monitor side effects, and compare it to existing treatments. This phase often involves multiple study sites and a diverse patient population to ensure the results are generalizable to a broader population. If the drug proves safe and effective in Phase III trials, the manufacturer can apply for regulatory approval to market it.
- Objective: To assess the drug’s efficacy and side effects.
- Participants: 100-300 patients.
- Duration: Up to 2 years.
Phase IV: Post-Marketing Surveillance
Once a drug has been approved for use and is on the market, Phase IV trials, also known as post-marketing surveillance, are conducted. These trials monitor the drug’s safety and efficacy in real-world conditions and among a larger population than was studied in the earlier clinical trial phases. Researchers collect data on any new side effects, drug interactions, or other safety concerns that may arise. This information updates drug labels, informs healthcare providers and patients, and guides future research.
- Objective: To confirm the drug’s effectiveness, monitor side effects, and compare it to commonly used treatments.
- Participants: 1,000-3,000 patients.
- Duration: 1-4 years.
Conclusion
Drug discovery and development require significant time, resources, and effort since it is a complicated and time-consuming process. Each stage of the process, from rational drug design and high-throughput screening to the different phases of clinical trials, plays a vital role in ensuring that new drugs are safe and effective. By comprehending this process, we can better understand the dedication and hard work required to bring new treatments to patients in need.
Disclaimer: This article is for informational purposes only and should not be taken as medical advice. Always consult with a healthcare professional before making any decisions related to medication or treatment.
Frequently Asked Questions
1. What is the purpose of drug discovery and development?
The purpose of drug discovery and development is to identify, design, and test potential new drugs with the aim of finding safe and effective treatments for various diseases and conditions.
2. What are the two main methods of drug design and screening?
Rational drug design and high-throughput screening are the two main drug design and screening methods.
3. What is the role of structure-activity relationships (SAR) in drug development?
The role of structure-activity relationships (SAR) is to help researchers understand how the chemical structure of a compound affects its biological activity. By analyzing SAR, researchers can modify a compound’s structure to improve its effectiveness, reduce side effects, or increase its ability to reach the target site in the body.
4. What are the four phases of clinical trials?
The four phases of clinical trials are Phase I (safety and dosage), Phase II (efficacy and side effects), Phase III (efficacy, monitoring, and comparison), and Phase IV (post-marketing surveillance).
5. Why are Phase IV trials important?
Phase IV trials, or post-marketing surveillance, are important because they monitor the safety and effectiveness of a drug in real-world conditions and among a larger population than was studied in earlier clinical trial phases. This helps identify new side effects, drug interactions, and other safety concerns that may arise, ultimately ensuring the drug remains safe and effective for its intended patient population.
Quiz on Drug Development
Medical Disclaimer
The medical information on this post is for general educational purposes only and is provided by Pharmacology Mentor. While we strive to keep content current and accurate, Pharmacology Mentor makes no representations or warranties, express or implied, regarding the completeness, accuracy, reliability, suitability, or availability of the post, the website, or any information, products, services, or related graphics for any purpose. This content is not a substitute for professional medical advice, diagnosis, or treatment; always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition and never disregard or delay seeking professional advice because of something you have read here. Reliance on any information provided is solely at your own risk.

