Introduction
Tuberculosis (TB) remains a major global health threat, caused by Mycobacterium tuberculosis (M.tb), a slow-growing, aerobic, acid-fast bacillus with a unique, lipid-rich cell wall conferring virulence and intrinsic drug resistance. Diagnosis relies on acid-fast stains, culture, PCR, and clinical criteria. TB is primarily pulmonary but can affect any organ. Increased risk is seen in immunosuppressed individuals, with airborne human-to-human transmission.
Microbiology and Pathogenesis: Key Points
- M. tuberculosis cell wall: High mycolic acid content → acid-fast, waxy barrier, resistant to many antibiotics and host defenses.
- Pathogenicity: Intracellular survival in macrophages, granuloma formation; latent and active disease states.
Classification and Mechanisms of Anti-Tubercular Drugs
Anti-TB drugs are divided into first-line, second-line, and new drugs based on clinical efficacy, toxicity, and role in multidrug-resistant TB (MDR-TB).
First-Line Drugs
| Drug | Mechanism of Action | Bactericidal/Static | Site of Action | Major Side Effects |
|---|---|---|---|---|
| Isoniazid | Inhibits mycolic acid synthesis (cell wall) via InhA and KasA inhibition; activated by KatG | Cidal (rapid growers); static (resting) | All (intra/extracellular) | Hepatotoxicity, neuropathy, rash |
| Rifampicin | Inhibits DNA-dependent RNA polymerase (β-subunit) → blocks RNA synthesis | Cidal | All (intra/extracellular) | Hepatotoxicity, orange discoloration, drug interactions |
| Ethambutol | Inhibits arabinogalactan synthesis via EmbB inhibition (cell wall) | Static | All | Optic neuritis, gout |
| Pyrazinamide | Prodrug → pyrazinoic acid inhibits fatty acid synthase I, disrupts membrane transport and energy | Cidal (in acidic environments) | Intracellular mycobacteria | Hepatotoxicity, hyperuricemia, arthralgia |
| Streptomycin | Inhibits protein synthesis (30S ribosome), misreading of mRNA | Cidal (extracellular) | Extracellular | Ototoxicity, nephrotoxicity, contraindicated in pregnancy |
References: Goodman & Gilman’s 13th Ed., Katzung 15th Ed., Lecturio.
Second-Line and New Anti-Tubercular Agents
| Class/Drug | Mechanism of Action | Clinical Role | Adverse Effects |
|---|---|---|---|
| Aminoglycosides (amikacin, kanamycin) | 30S ribosome inhibitor (protein synthesis) | MDR-TB, XDR-TB | Nephro/ototoxicity |
| Capreomycin | 70S ribosome, initiator complex inhibitor | MDR-TB, XDR-TB | Similar to aminoglycosides |
| Fluoroquinolones (levofloxacin, moxifloxacin) | Inhibits DNA gyrase/topoisomerase IV | MDR-TB; MAC, XDR-TB | Tendinopathy, CNS effects |
| Ethionamide | Inhibits mycolic acid synthesis (like INH) | MDR-TB, leprosy | GI, neurotoxicity, hepatotoxicity |
| Cycloserine | Inhibits cell wall peptide synthesis (D-alanine racemase, ligase) | MDR-TB, neuropsychiatric | Neurotoxicity (depression, seizures) |
| PAS | Inhibits folic acid synthesis | MDR-TB | GI, liver, thyroid dysfunction |
| Linezolid | 50S ribosome inhibitor (protein synthesis) | MDR/XDR-TB, MAC | Myelosuppression, neuropathy |
| Clofazimine | DNA binding, membrane disruption, ROS generation | MDR/XDR-TB, leprosy | Skin discoloration, GI effects |
| Bedaquiline | ATP synthase inhibitor (energy metabolism) | MDR/XDR-TB (core new drug) | QT prolongation, hepatotoxicity |
| Delamanid | Inhibits mycolic acid synthesis, ATP generation (nitroimidazole group) | MDR/XDR-TB (core new drug) | QT prolongation |
| Pretomanid | Converted to reactive nitrogen species—DNA/protein/membrane damage | MDR/XDR-TB (combo regimens) | GI, QT prolongation |
References: Goodman & Gilman’s 13th Ed., WHO 2022 guidelines, newtbdrugs.org.
Pharmacology Details of Core Drugs
Isoniazid (INH)
- Pharmacokinetics: Well absorbed orally; distributed to all tissues including CSF. Metabolized by hepatic acetylation (fast/slow acetylators – genetic polymorphism). Excreted in urine.
- Adverse Effects: Hepatotoxicity (age-related), peripheral neuropathy (especially slow acetylators, diabetics, malnourished), treated/prevented with pyridoxine.
- Clinical Pearls: Backbone of all regimens, resistance via KatG/inhA mutations, always used in combination.
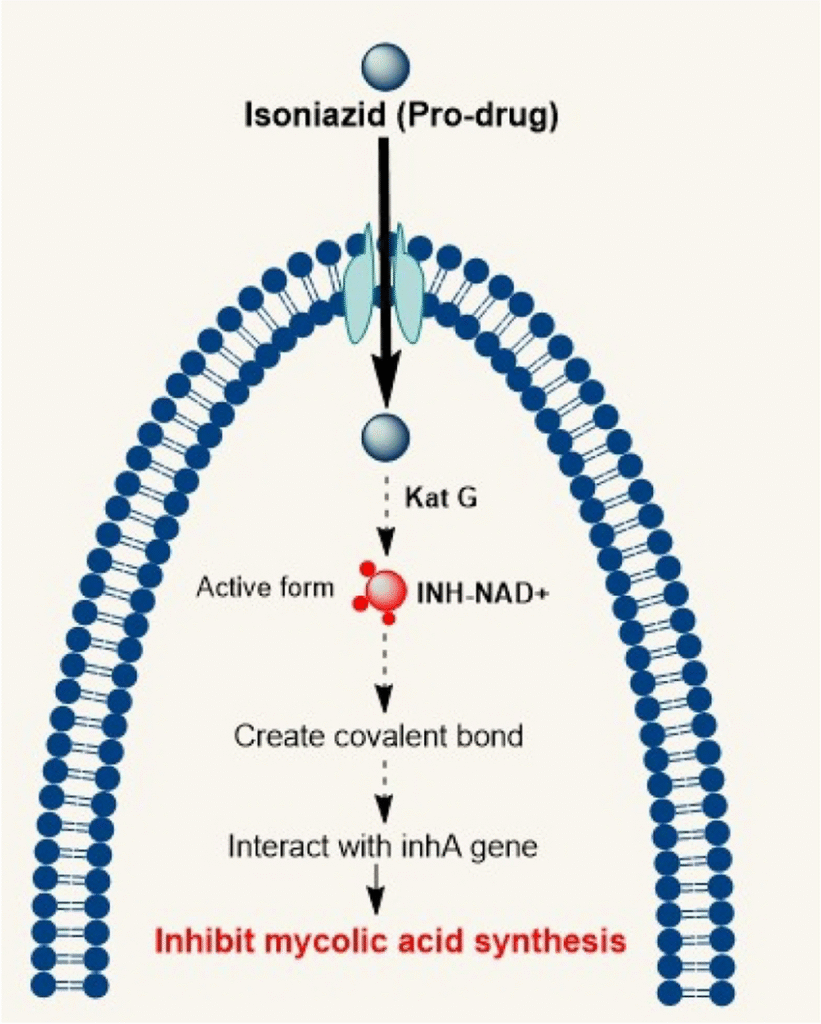
Source: Antitubercular drugs: possible role of natural products acting as antituberculosis medication in overcoming drug resistance and drug-induced hepatotoxicity – Scientific Figure on ResearchGate. Available from: https://www.researchgate.net/figure/Mechanism-of-action-of-INH-on-Mycobacterium-tuberculosis_fig1_373657618 [accessed 8 Nov 2025]
Rifampicin (RIF)
- Pharmacodynamics: Oral, excellent tissue penetration, including CNS and intracellular compartments.
- Adverse Effects: Hepatotoxicity, potent CYP450 induction—multiple drug interactions, orange-red urine/fluids, thrombocytopenia.
- Clinical Pearls: Indispensable in TB, leprosy, MAC, and as a prophylactic. Resistance due to rpoB mutation.
Pyrazinamide (PZA)
- Pharmacokinetics: Absorbed orally; distributed widely. Metabolized hepatically; excreted renally.
- Adverse Effects: Hepatotoxicity, hyperuricemia, arthralgia.
- Pearl: Essential for intensive phase, sterilizing action in acidic environments.
Ethambutol (EMB)
- Pharmacodynamics: Oral admin. Bacteriostatic. 20% fecal, 50% renal excretion.
- Adverse Effects: Optic neuritis (monitor vision), rare hepatotoxicity.
- Pearl: Included to prevent resistance during initial phase.
Streptomycin
- Administration: IM only, not oral.
- Adverse Effects: Ototoxicity, nephrotoxicity.
- Pearl: Used less frequently—MDR/XDR regimen adjunct, contraindicated in pregnancy.
New TB Drugs: Bedaquiline, Delamanid, Pretomanid
- Bedaquiline and delamanid are now WHO Group A core drugs for MDR/XDR regimens, typically used with linezolid and other agents for 6–9 month oral short-course regimens—improving cure rates, decreasing toxicity, and offering more convenient therapy.
WHO/India National TB Guidelines: Treatment Algorithms
Drug-Sensitive TB (Category I)
- Intensive Phase: 2 months—HRZE (isoniazid, rifampicin, pyrazinamide, ethambutol)
- Continuation Phase: 4 months—HR (isoniazid, rifampicin)
- Total Duration: 6 months minimum
Category II (Previously treated/relapse):
- Intensive Phase: 2(HRZES) / 1(HRZE)
- Continuation Phase: 5(HRE)
Drug-Resistant TB (MDR/XDR)
- MDR-TB (18–20 month regimen): 5 active drugs minimum: pyrazinamide, a fluoroquinolone (e.g., levofloxacin), an injectable aminoglycoside (e.g., amikacin/kanamycin/capreomycin), and a core drug (bedaquiline/delamanid).
- WHO Short-Course Regimen (BPaLM/BPaL): Bedaquiline, pretomanid, linezolid ± moxifloxacin; 6–9 months
- XDR-TB: Fewer options—combination regimens based on susceptibility (often includes all new drugs).
Adverse Effects & Monitoring
| Drug | Common Side Effects | Key Monitoring |
|---|---|---|
| Isoniazid | Hepatitis, neuropathy | LFTs, neuropathy |
| Rifampicin | Hepatitis, rash, drug interaction | LFTs, DDI review |
| Pyrazinamide | Arthritis, hepatitis, hyperuricemia | LFTs, serum uric acid |
| Ethambutol | Optic neuritis, rash | Baseline and periodic vision |
| Streptomycin | Ototoxicity, nephrotoxicity | Hearing, renal function |
| Injectable aminoglycosides | Nephro-, oto-toxicity | Renal, hearing |
| Fluoroquinolones | Tendinopathy, CNS | Tendon/CNS symptoms |
| Bedaquiline | QT prolongation, hepatotoxicity | ECG, LFTs |
| Delamanid | QT prolongation | ECG |
| Pretomanid | GI, QT prolongation | ECG, GI symptoms |
References: Goodman & Gilman’s 13th Ed., newtbdrugs.org, WHO.
Treatment of Atypical Mycobacterial Infections
- MAC (Mycobacterium avium complex): Prophylaxis: clarithromycin/azithromycin for low CD4 (<50) HIV patients.
- REC regimen: Rifabutin + ethambutol + clarithromycin/azithromycin.
- Quinolones and aminoglycosides: Additional roles; use in resistant cases.
Key Principles of Anti-TB Therapy
- Always use multiple drugs—monotherapy leads to resistance
- Directly Observed Therapy (DOTS): Ensures compliance, prevents resistance
- Adjust therapy based on susceptibility, previous treatment, and patient tolerance
Summary Tables
Main Characteristics of First-Line Drugs
| Drug | Dose (adult) | Main Mechanism | Critical Adverse Effects |
|---|---|---|---|
| Isoniazid | 5 mg/kg | InhA mycolic acid inhibition | Hepatitis, neuropathy |
| Rifampicin | 10 mg/kg | RNA polymerase inhibition | Hepatitis, CYP induction |
| Pyrazinamide | 20–25 mg/kg | Fatty acid synthase inhibition | Hepatitis, hyperuricemia |
| Ethambutol | 15–25 mg/kg | Arabinosyl transferase inhibition | Optic neuritis |
| Streptomycin | 15 mg/kg (IM) | 30S ribosome inhibition | Ototoxicity, nephrotoxicity |
New Drug Table (MDR/XDR)
| Drug | Group/Class | Standard Adult Dose | Principal Use | Side Effects/Monitoring |
|---|---|---|---|---|
| Bedaquiline | Diarylquinoline | 400 mg daily x 2w, then 200 mg 3x/wk | MDR/XDR core drug | QT, LFTs |
| Delamanid | Nitroimidazole | 100 mg bid | MDR/XDR core drug | QT, LFTs |
| Pretomanid | Nitroimidazole | 200 mg daily (in BPaL) | XDR regimens | QT, GI, LFTs |
References & Further Reading
- Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 13th Edition
- Katzung BG, Trevor AJ. Basic & Clinical Pharmacology, 15th Edition
- WHO TB Treatment Guidelines, 2022 Update
- newtbdrugs.org: Bedaquiline & Delamanid/Drug Pipeline
- Lecturio: Antimycobacterial Drugs
- Frontiers: New TB Pipeline Review
- CUTM Courseware: Antitubercular Drug
- MSF Medical Guidelines: Drug-resistant TB
- Indian National Guidelines, tbcindia.mohfw.gov.in
- EndTB.org Guide for New TB Drugs
📚 AI Pharma Quiz Generator
🎉 Quiz Results
Medical Disclaimer
The medical information on this post is for general educational purposes only and is provided by Pharmacology Mentor. While we strive to keep content current and accurate, Pharmacology Mentor makes no representations or warranties, express or implied, regarding the completeness, accuracy, reliability, suitability, or availability of the post, the website, or any information, products, services, or related graphics for any purpose. This content is not a substitute for professional medical advice, diagnosis, or treatment; always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition and never disregard or delay seeking professional advice because of something you have read here. Reliance on any information provided is solely at your own risk.

