INTRODUCTION
Aminoglycoside antibiotics are a class of potent, broad-spectrum bactericidal agents that have played a critical role in the management of serious bacterial infections for decades. First derived from microbial sources in the mid-20th century, these compounds quickly became indispensable, especially for treating infections caused by aerobic Gram-negative bacteria. Despite the rise of newer antimicrobial classes, aminoglycosides remain particularly valuable in hospital settings for severe or multidrug-resistant (MDR) pathogens, often in combination with other antibiotics.
Although powerful, aminoglycosides require prudent use due to notable potential toxicities, particularly nephrotoxicity and ototoxicity. Their narrow therapeutic index mandates careful dosing and monitoring. In this review, we will explore the structure, classification, mechanism of action, pharmacokinetics, pharmacodynamics, clinical applications, adverse effects, resistance mechanisms, and modern approaches to optimizing aminoglycoside therapy.
CHEMICAL STRUCTURE AND CLASSIFICATION
Basic Chemical Structure
Aminoglycosides are composed of an amino-modified glycoside (sugar).
Typically, they feature:
• A central hexose ring (streptamine, 2-deoxystreptamine, or streptidine).
• Multiple amino (–NH2) groups and hydroxyl (–OH) groups.
• Glycosidic bonds linking additional sugar moieties.
The large number of polar groups confers high water solubility and poor oral absorption, which has important implications for their pharmacokinetics. Because of their structure, aminoglycosides are mostly administered parenterally (intravenous or intramuscular) for systemic therapy.
Examples of Clinically Relevant Aminoglycosides
• Streptomycin: One of the earliest discovered. Historically used for tuberculosis (TB) treatment and plague (Yersinia pestis).
• Gentamicin: A mainstay for severe Gram-negative infections, typically utilized in hospital settings.
• Tobramycin: Similar spectrum to gentamicin but slightly superior against Pseudomonas aeruginosa. Also used in inhaled formulations for cystic fibrosis infections.
• Amikacin: Synthetic derivative designed to resist many common aminoglycoside-inactivating enzymes. It has a wider spectrum, often active against gentamicin- or tobramycin-resistant organisms.
• Neomycin: Largely restricted to topical or oral bowel prep uses due to systemic toxicity.
• Kanamycin: Historically used for TB and Gram-negative infections; less commonly employed today.
• Plazomicin: A newer aminoglycoside with modifications aimed at overcoming most known aminoglycoside-resistant mechanisms. Approved for complicated urinary tract infections (cUTIs) and showing promise against emerging resistant pathogens.
Classification Approach
A typical classification scheme groups aminoglycosides by their source and structural features (e.g., those derived from Micromonospora vs. Streptomyces). Clinically, however, agents are commonly differentiated based on their specific activity or resistance to inactivating enzymes (e.g., amikacin is less susceptible to many aminoglycoside-modifying enzymes). Regardless of classification, they share common pharmacological properties.
MECHANISM OF ACTION
Targeting Bacterial Protein Synthesis
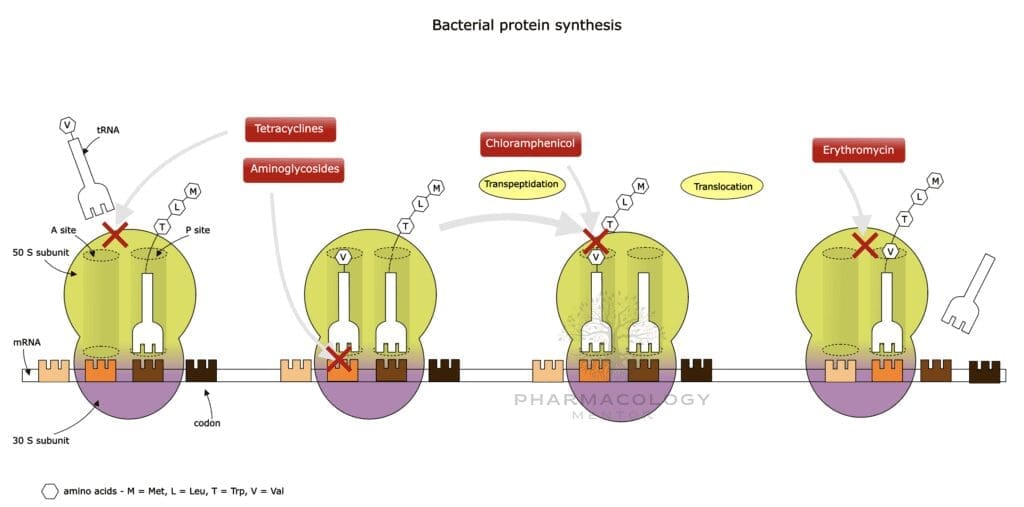
Aminoglycosides enter the bacterial cell through a stepwise process: • An initial electrostatic interaction with negatively charged lipopolysaccharides (LPS) in the outer membrane of Gram-negative bacteria.
• Uptake across the inner membrane is an oxygen-dependent process (often termed “energy-dependent phase I”). This requirement for oxygen partially explains why aminoglycosides have limited activity against anaerobic bacteria.
Once inside, aminoglycosides bind irreversibly to the 30S ribosomal subunit. They interfere with bacterial protein synthesis by:
• Blocking the initiation complex formation.
• Inducing misreading of mRNA, causing the incorporation of incorrect amino acids into nascent polypeptides.
• Causing premature termination of protein synthesis.
This disruption severely compromises bacterial viability, conferring a rapid bactericidal effect. Notably, the effect is concentration-dependent: higher concentrations lead to more rapid killing.
Concentration-Dependent Killing and Post-Antibiotic Effect
Aminoglycosides display concentration-dependent killing, meaning higher peak plasma concentrations enhance their bactericidal activity. They also exhibit a post-antibiotic effect (PAE), where bacterial growth remains suppressed for a certain period even after serum drug concentrations fall below the minimum inhibitory concentration (MIC). This phenomenon underpins the rationale behind once-daily dosing strategies in some clinical settings.
PHARMACOKINETICS
Absorption
• Oral Bioavailability: Extremely poor (less than 1%) due to high polarity and limited GI membrane permeability. Hence, aminoglycosides are typically given parenterally (IV or IM) for systemic effect.
• Topical Use: Some aminoglycosides (e.g., neomycin) are used on the skin or ocular surfaces, with minimal systemic absorption when the integrity of the skin or mucosa is intact.
Distribution
• Volume of Distribution (Vd): Relatively small (restricted primarily to extracellular fluid). Typical Vd is around 0.25 L/kg in healthy adults but may vary with body fluid changes (e.g., ascites, edema, dehydration).
• Tissue Penetration: Limited penetration into adipose tissue and cerebrospinal fluid (CSF). For central nervous system infections, especially in neonates, intrathecal or intraventricular administration might be used.
• Protein Binding: Negligible, generally less than 10%.
Metabolism
• Minimal Metabolism: Aminoglycosides largely remain unmodified in the body. A small fraction may undergo partial inactivation in the renal tubules, but hepatic metabolism is not a major pathway.
Excretion
• Renal Elimination: Excretion by glomerular filtration in the kidneys as unchanged drug. This dependence on renal clearance underlies a significant risk of accumulation and toxicity in cases of renal impairment.
• Half-Life: In patients with normal renal function, the half-life ranges from about 2 to 3 hours but can extend dramatically in renal dysfunction or in neonates.
Implications for Dosing
Because aminoglycosides rely on renal excretion, dosing intervals and total daily doses require adjustment in patients with decreased renal function to avoid accumulation and toxicity. Monitoring of serum concentrations (peak and trough levels) is often standard practice to optimize therapy while minimizing adverse effects.
PHARMACODYNAMICS
Concentration-Dependent Bactericidal Activity
A central pharmacodynamic principle of aminoglycosides is their concentration-dependent killing:
• Peak/MIC Ratio: The ratio of peak plasma concentration to the minimum inhibitory concentration correlates closely with bactericidal efficacy.
• “Once-Daily” or Extended-Interval Dosing: This approach aims for higher, more potent peaks, followed by a drug-free interval that can reduce toxicity risk (owing to saturable uptake by renal tubular cells).
Post-Antibiotic Effect (PAE)
The PAE can last several hours, sustaining inhibitory or sublethal damage to bacteria even when the drug level dips below the MIC. This characteristic further supports extended-dose intervals, as it provides a buffer period for the host to eliminate partially compromised bacteria.
Synergy with Other Antibiotics
• Beta-Lactams: Combining aminoglycosides with beta-lactams can produce synergistic killing, especially for enterococci and certain Gram-negative bacilli (e.g., Pseudomonas aeruginosa). Beta-lactams disrupt the cell wall, augmenting aminoglycoside entry.
• Glycopeptides: A similar rationale applies when combined with vancomycin, for instance in treating complicated endocarditis caused by enterococci.
However, physical in vitro admixture of aminoglycosides and some beta-lactams (in the same IV bag or line) can result in inactivation, so separate administration lines or intervals are recommended.
SPECTRUM OF ACTIVITY
Gram-Negative Bacteria
Aminoglycosides are particularly active against many aerobic Gram-negative rods, such as:
• Enterobacteriaceae (Escherichia coli, Klebsiella pneumoniae, Proteus spp., etc.).
• Pseudomonas aeruginosa (tobramycin and amikacin often used).
• Acinetobacter spp. (amikacin often has good activity, but resistance patterns vary).
Gram-Positive Bacteria
Their utility for Gram-positive organisms is often limited by relatively lower potency. However, certain scenarios benefit from synergy:
• Staphylococci (e.g., synergy with beta-lactams for staphylococcal endocarditis).
• Streptococci or Enterococci (particularly synergy for endocarditis).
They are rarely used alone for Gram-positive coverage, as effective alternatives with fewer toxicities exist. Nonetheless, synergy remains a critical concept in severe infections like endocarditis.
Atypical Pathogens
Aminoglycosides generally have minimal activity against anaerobes or intracellular pathogens (e.g., Mycoplasma, Chlamydia) due to the oxygen-requiring uptake mechanism.
Mycobacteria
Streptomycin, amikacin, and kanamycin feature in some tuberculosis regimens (particularly for resistant TB strains) and treatment of nontuberculous mycobacteria. However, their role is typically adjunctive.
THERAPEUTIC USES
Serious Gram-Negative Infections
Aminoglycosides are frequently employed in hospitalized patients with severe systemic infections:
• Sepsis, bacteremia, or pneumonia caused by multidrug-resistant Gram-negative organisms.
• Febrile neutropenia in hematologic malignancy patients, often combined with beta-lactams.
• Hospital-acquired or ventilator-associated pneumonia, particularly when P. aeruginosa or other resistant Gram-negatives are suspected.
Combination Therapy for Gram-Positive Infections
• Endocarditis: Gentamicin (or occasionally streptomycin) in synergy with a penicillin-like agent or vancomycin for treating enterococcal or streptococcal endocarditis.
• Prosthetic Joint Infections or Osteomyelitis: Short-course aminoglycoside synergy, although toxicity concerns often limit prolonged use.
Tuberculosis and Mycobacterial Diseases
• Streptomycin was historically a first-line TB agent but is less commonly used now due to resistance and toxicity concerns.
• Amikacin may be employed in drug-resistant TB regimens or for atypical mycobacterial infections.
Topical/Local Uses
• Neomycin or gentamicin ointments/creams for skin infections.
• Tobramycin eye drops for ocular infections (e.g., bacterial conjunctivitis).
• Paromomycin is used orally for intestinal parasites (e.g., amoebiasis) or in topical form for cutaneous leishmaniasis.
Inhaled Formulations
• Inhaled tobramycin is integral in managing chronic Pseudomonas infections in cystic fibrosis, improving lung function and reducing exacerbations.
DOSING AND ADMINISTRATION
Traditional Multiple-Daily Dosing
Historically, aminoglycosides were given in two or three divided doses per day. This schedule aimed to maintain adequate plasma levels but carries an increased risk for accumulation in renal cortical cells and inner ear fluid unless trough levels are carefully monitored.
Once-Daily (Extended-Interval) Dosing
Many institutions have adopted once-daily dosing (5–7 mg/kg gentamicin or tobramycin, or 15–20 mg/kg amikacin) for severe infections in patients with stable renal function. Advantages include: • Achieving high peak levels that maximize bactericidal effect.
• Allowing a drug-free period that may reduce toxicity (as aminoglycoside uptake in renal and cochlear cells is saturable).
• Simplifying administration and decreasing the need for frequent levels.
Contraindications to once-daily dosing might include pregnancy, severe renal insufficiency, or synergy for endocarditis (where smaller, more frequent doses may be preferred for maintaining consistent synergy).
Monitoring Serum Levels
• Peak Levels: Typically measured ~30 minutes after the end of an IV infusion (for multiple-daily dosing). For once-daily regimens, peak levels are usually not routinely measured unless needed to confirm efficacy.
• Trough Levels: Most critical to evaluate risk of toxicity. For multiple-daily dosing, a trough should be <1 µg/mL (gentamicin/tobramycin) or <5 µg/mL (amikacin).
• Random Levels and Nomograms: Some institutions use Hartford or similar nomograms after a single high dose, measuring serum concentration at 6–14 hours post-dose to determine subsequent dosing intervals.
Renal Function Adjustments
GFR estimation is critical to guide dosing adjustments. In patients with renal insufficiency, total daily dose or frequency may require modification to avoid dangerous accumulation. Therapeutic drug monitoring is essential in these scenarios.
ADVERSE EFFECTS, TOXICITY, AND SAFETY CONCERNS
Nephrotoxicity
• Mechanism: Aminoglycosides are taken up by proximal tubular cells in the kidney. They can induce tubular cell necrosis.
• Clinical Presentation: Elevated serum creatinine, reduced urine output, potential electrolyte imbalances. Typically occurs after several days of therapy but can manifest earlier in predisposed individuals (e.g., hypovolemia, co-administration of other nephrotoxic drugs).
• Reversibility: Often reversible if recognized early and therapy is stopped or adjusted. However, acute kidney injury (AKI) can be severe and occasionally irreversible.
Ototoxicity
• Cochleotoxicity: Presents as hearing loss (often high-frequency first).
• Vestibulotoxicity: Manifests as vertigo, ataxia, and balance disturbances.
• Mechanism: Aminoglycosides accumulate in the endolymph and perilymph of the inner ear, damaging hair cells.
• Onset: May be delayed and can progress even after discontinuation of therapy. Hearing loss is often irreversible.
Other Adverse Effects
• Neuromuscular Blockade: At high levels, aminoglycosides can interfere with neuromuscular transmission, causing weakness or respiratory depression. This risk is heightened with concurrent neuromuscular-blocking agents or in conditions like myasthenia gravis.
• Dermatologic Reactions: Rash or rarely anaphylaxis (though relatively uncommon).
• Rare allergic responses: Cross-allergenicity between different aminoglycosides may exist but is not as widely documented as with other antibiotic classes.
Risk Factors for Toxicity
• Prolonged therapy, high cumulative dose.
• Pre-existing renal dysfunction.
• Concomitant use of other nephrotoxic agents (e.g., vancomycin, NSAIDs, contrast media).
• Advanced age or dehydration.
• Elevated trough concentrations or poor monitoring practices.
RESISTANCE MECHANISMS
Aminoglycoside resistance poses a significant clinical challenge, arising mainly through three mechanisms:
Enzymatic Modification
The most common route involves aminoglycoside-modifying enzymes (AMEs) such as:
• Acetyltransferases (AAC)
• Phosphotransferases (APH)
• Nucleotidyltransferases (ANT)
These enzymes alter specific sites on the aminoglycoside molecule, reducing its affinity for the 30S ribosome.
Reduced Uptake or Enhanced Efflux
Any changes that decrease the drug’s penetration into the bacterial cytoplasm can confer resistance:
• Alterations in outer membrane porin channels.
• Modified proton-motive force diminishing active transport.
• Efflux pumps that actively expel aminoglycosides.
Target Site Mutation
Although less frequent than enzymatic modification, mutations in the 16S rRNA of the 30S ribosomal subunit can impair drug binding.
Clinical Implications
• Amikacin and plazomicin are less susceptible to many AMEs, making them viable options for organisms resistant to gentamicin or tobramycin.
• Combination therapy (with, for instance, a beta-lactam) can help overcome partial resistance in certain scenarios.
STRATEGIES TO MINIMIZE TOXICITY AND RESISTANCE
Optimal Dosing Approaches
• High-dose, extended-interval regimens can maximize bactericidal effect while allowing lower trough concentrations and reducing toxicity.
• Therapeutic drug monitoring is invaluable for tailoring individual doses.
Limiting Treatment Duration
Since the risk of toxicity increases significantly with extended use, guidelines emphasize limiting aminoglycoside therapy to the shortest effective duration. Once culture and susceptibility results become available, clinicians may de-escalate therapy.
Stewardship Programs
Antimicrobial stewardship initiatives ensure that aminoglycosides are used judiciously:
• Prescribing protocols or automatic stop orders.
• Education on synergy-based usage and appropriate prophylactic indications.
• Automated dosing calculators or pharmacist-led therapeutic drug monitoring clinics.
Novel Agents and Combinations
• Plazomicin is an example of a newer-generation aminoglycoside engineered to evade many AMEs. It can be particularly useful in carbapenem-resistant Enterobacteriaceae (CRE) infections when other options are limited.
• Adjunctive therapies (such as beta-lactamase inhibitors or colistin) can further augment bacterial kill or circumvent specific resistance mechanisms.
SPECIAL POPULATIONS AND CONSIDERATIONS
Renal Impairment
• Aminoglycosides require careful dose adjustment and vigilant monitoring.
• Extended-interval dosing can still be utilized in stable chronic kidney disease, but intervals may need to be prolonged (e.g., 36 or 48 hours).
Pediatrics and Neonates
• Pharmacokinetics differ significantly, with neonates having an increased volume of distribution and immature renal function.
• Dosing frequency and intervals may be adjusted (often once-daily or every 12 hours with close monitoring).
Pregnancy and Lactation
• Aminoglycosides cross the placenta and may pose a risk of congenital deafness. Nevertheless, they may be used if no suitable alternative exists, with strict follow-up.
• Pass into breast milk in small quantities. While not typically an absolute contraindication, caution is advised.
Obesity
• Aminoglycoside dosing in obese patients can be challenging. Adjusted body weight is often used to avoid overdosing, since these medications do not distribute extensively into adipose tissue.
EMERGING THERAPIES AND RESEARCH DIRECTIONS
Next-Generation Agents
• Plazomicin demonstrates potent activity against a spectrum of resistant Gram-negative bacilli, including Klebsiella pneumoniae carbapenemase (KPC)-producing strains. Clinicians await further real-world data on resistance evolution.
Formulation Advances
• Liposomal or nanoparticle-based formulations might be explored to reduce toxicity by modulating renal accumulation.
• Inhaled preparations beyond tobramycin (e.g., amikacin liposome inhalation) are being studied for lung infections, particularly in cystic fibrosis or bronchiectasis.
Adjunctive Protectants
• Research on potential otoprotectants or nephroprotectants (such as adjuvant agents that could mitigate aminoglycoside-induced oxidative injury in tissues) is ongoing.
• Genetic testing for predisposition to aminoglycoside ototoxicity (e.g., mitochondrial m.1555A>G mutation) might inform personalized medicine approaches.
Microbial Genomics
• Whole-genome sequencing for resistant bacteria elucidates novel AMEs and resistance determinants, informing the design of future aminoglycoside derivatives or combination regimens.
CLINICAL PEARLS AND SUMMARY
Key Takeaways
• Aminoglycosides are rapid, concentration-dependent bactericidal agents chiefly used against Gram-negative bacteria. They also exhibit synergy with cell wall-active antibiotics against certain Gram-positive cocci.
• Nephrotoxicity and ototoxicity are the major dose-limiting adverse effects. Regular monitoring of serum levels, renal function, and hearing is crucial.
• Extended-interval dosing regimens exploit the drug’s pharmacokinetic-pharmacodynamic advantages to potentially reduce toxicity while enhancing efficacy.
• Resistance arises primarily via enzymatic drug modification, reduced uptake, and ribosomal target mutations. Newer agents (e.g., plazomicin) aim to circumvent these mechanisms.
• Appropriate use, guided by stewardship principles, is essential in preventing the spread of resistance and conserving the utility of aminoglycosides.
Place in Contemporary Therapy
Despite recognized toxicity risks, aminoglycosides remain vital in the combat against resistant Gram-negative infections, especially in critically ill patients. Methods to lower toxicity (like single-dose regimens, close monitoring, synergy) allow clinicians to harness their potent bactericidal capabilities. Emerging research promises further refinements in safety and spectrum, ensuring that aminoglycosides continue to be an important component of our antimicrobial armamentarium.
CONCLUSION
Aminoglycosides have a storied history within the field of infectious disease treatment. Their robust antimicrobial effects and synergy with beta-lactams or glycopeptides makes them particularly effective in severe or life-threatening infections, notably with resistant Gram-negative organisms. Treading carefully around their narrow therapeutic index is of utmost importance: diligent dosing, vigilant monitoring, and prompt recognition of adverse effects are necessary to derive maximum clinical benefit while minimizing nephrotoxicity and ototoxicity.
In an era of escalating antibiotic resistance, aminoglycosides continue to play an important role, often as part of well-coordinated combination regimens. The development of newer agents like plazomicin illustrates the continued relevance and adaptability of this antibiotic class. Antimicrobial stewardship, research into novel dosing strategies, improved formulations, and adjunctive protectants will shape the future utilization of aminoglycosides, ensuring their utility for future generations in the everlasting quest to control bacterial infections.
Medical Disclaimer
The medical information on this post is for general educational purposes only and is provided by Pharmacology Mentor. While we strive to keep content current and accurate, Pharmacology Mentor makes no representations or warranties, express or implied, regarding the completeness, accuracy, reliability, suitability, or availability of the post, the website, or any information, products, services, or related graphics for any purpose. This content is not a substitute for professional medical advice, diagnosis, or treatment; always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition and never disregard or delay seeking professional advice because of something you have read here. Reliance on any information provided is solely at your own risk.

