Introduction
Co-trimoxazole—a combination of sulfamethoxazole and trimethoprim—has been a cornerstone antibacterial agent for decades. The synergy between these two drugs proves highly effective against a broad range of Gram-positive and Gram-negative microorganisms. First introduced in the 1960s, co-trimoxazole quickly rose to prominence, offering a potent yet pragmatic solution for infections spanning the respiratory, urinary, and gastrointestinal tracts, among others (Goodman & Gilman, 2018).
As antibiotic resistance poses an increasing threat, co-trimoxazole retains a vital place in guidelines for managing specific opportunistic infections (notably those seen in immunocompromised states such as HIV/AIDS) and for selected prophylactic indications. This comprehensive overview discusses co-trimoxazole’s chemical properties, mechanisms of action, pharmacokinetics, clinical applications, adverse effects, and resistance patterns. Drawing mainly from authoritative sources like “Goodman & Gilman’s The Pharmacological Basis of Therapeutics,” “Katzung’s Basic & Clinical Pharmacology,” and “Rang & Dale’s Pharmacology,” it aims to synthesize the drug’s pharmacological profile for healthcare professionals, researchers, and students seeking in-depth knowledge on an essential antibacterial agent.
Composition and Rationale of Combination
Sulfamethoxazole
A sulfonamide antibiotic, sulfamethoxazole exerts bacteriostatic activity by inhibiting the enzyme dihydropteroate synthase, which participates in folic acid synthesis for bacterial growth. It is structurally similar to para-aminobenzoic acid (PABA) and competes with PABA to block dihydropteroate synthase, preventing the conversion of PABA into dihydropteroic acid, a folate precursor (Katzung, 2020).
Trimethoprim
Trimethoprim selectively inhibits dihydrofolate reductase (DHFR), another key enzyme in the folate pathway. By blocking DHFR, trimethoprim impedes the conversion of dihydrofolate (DHF) to tetrahydrofolate (THF), thus depleting the pool of folate derivatives essential for synthesizing nucleic acids. Compared to mammalian DHFR, bacterial DHFR has a higher affinity for trimethoprim, conferring a measure of selective toxicity (Rang & Dale, 2019).
Synergistic Relationship
When used together in a fixed ratio of 5 parts sulfamethoxazole to 1 part trimethoprim (by weight), these agents produce a sequential blockade of folate metabolism at two distinct steps—often described as a “two-hit” approach. This synergy achieves bactericidal activity against many bacteria (whereas each agent alone is typically bacteriostatic). This combined effect underpins co-trimoxazole’s broad efficacy, making it a valuable option for a variety of infectious diseases (Goodman & Gilman, 2018).
Mechanism of Action
Folic Acid Synthesis Inhibition
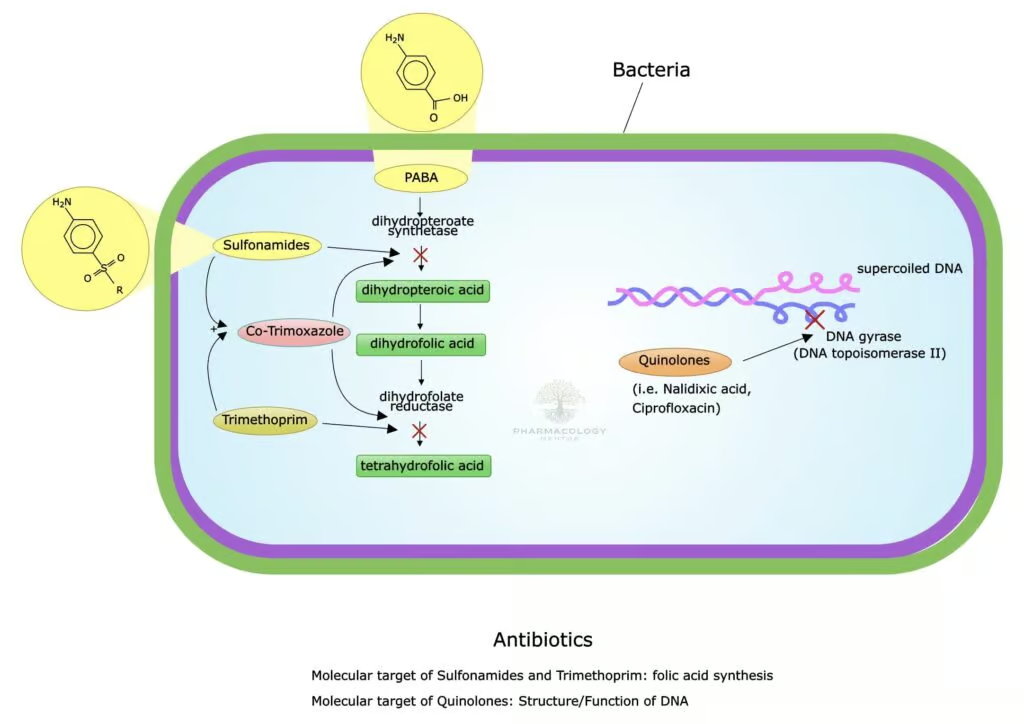
Bacteria conventionally synthesize their own folate de novo. The process begins with the incorporation of PABA to form dihydropteroic acid, a reaction catalyzed by dihydropteroate synthase. Sulfamethoxazole competes with PABA, halting dihydropteroic acid production. Next, the dihydropteroic acid is converted to dihydrofolic acid (DHF) and subsequently to the active tetrahydrofolate (THF) form via dihydrofolate reductase. Trimethoprim blocks that specific reductive step. Thus, each agent tackles a different sequence in THF production, culminating in severe folate deficiency and stalling DNA synthesis within the microbial cell (Katzung, 2020).
Bactericidal Synergy
Individually, sulfamethoxazole and trimethoprim can be bacteriostatic, but in combination, they often act bactericidal. This synergy stems from drastically lowered folate availability within bacteria, preventing them from producing essential nucleic acids for replication and survival (Rang & Dale, 2019).
Spectrum of Activity
Gram-Positive Bacteria
- Staphylococcus aureus (including community-acquired MRSA strains): Co-trimoxazole is frequently used for mild-to-moderate skin infections and community-associated MRSA coverage, though some regions face rising resistance.
- Streptococcus pneumoniae: Often susceptible, though some strains may exhibit partial resistance.
- Listeria monocytogenes: Co-trimoxazole can be an alternative to ampicillin in managing listeriosis if patients have penicillin allergy (Goodman & Gilman, 2018).
Gram-Negative Bacteria
- Enterobacteriaceae (Escherichia coli, Klebsiella, Enterobacter): Common usage for urinary tract infections, albeit with region-specific resistance patterns that clinicians must monitor.
- Haemophilus influenzae, Moraxella catarrhalis: Often susceptible, relevant for certain respiratory infections.
- Salmonella, Shigella species: Historically effective for GI infections such as bacillary dysentery and enteric fevers, though local resistance must be evaluated (Katzung, 2020).
Opportunistic Organisms
- Pneumocystis jirovecii (previously Pneumocystis carinii): Co-trimoxazole is the first-line prophylaxis and treatment for Pneumocystis pneumonia (PCP) in immunocompromised patients (e.g., HIV/AIDS).
- Toxoplasma gondii: Used adjunctively for toxoplasmosis prophylaxis and therapy in immunosuppressed populations.
- Nocardia asteroides: Treatment of choice for nocardiosis (Rang & Dale, 2019).
Atypical or Poor Coverage
- Generally poor efficacy against Pseudomonas aeruginosa and most anaerobes.
- Not recommended for Mycoplasma or viruses.
- Some strains of staphylococci and enterococci, as well as emerging resistant E. coli, necessitate alternative regimens (Goodman & Gilman, 2018).
Pharmacokinetics
Absorption
Following oral administration, co-trimoxazole (sulfamethoxazole + trimethoprim) is well absorbed from the GI tract, with bioavailability generally around 85%-90%. Trimethoprim is somewhat more lipophilic, achieving higher tissue penetration (Katzung, 2020).
Distribution
Both components distribute widely, including lungs, kidney tissue, prostate gland, and CSF to varying degrees. Trimethoprim typically has a higher volume of distribution. This broad tissue penetration underlies co-trimoxazole’s efficacy in respiratory, urinary, and opportunistic infections.
- Protein Binding: Sulfamethoxazole is moderately bound (~70%), while trimethoprim is ~40% bound, allowing a significant free fraction to engage pathogens (Rang & Dale, 2019).
Metabolism
- Sulfamethoxazole undergoes hepatic acetylation and glucuronidation, creating inactive metabolites.
- Trimethoprim experiences hepatic oxidation or hydroxylation, forming metabolites with minimal antibacterial activity (Goodman & Gilman, 2018).
Elimination
Primary excretion occurs via the kidneys through both glomerular filtration and tubular secretion. A portion of each drug is excreted unchanged in urine, particularly important in urinary tract infections. Half-lives are about 10–11 hours for sulfamethoxazole and 8–10 hours for trimethoprim in individuals with normal renal function (Katzung, 2020).
Dosing Regimens
Standard adult dosing typically references trimethoprim content (e.g., “TMP 160 mg + SMX 800 mg” twice daily). In severe infections (Pneumocystis pneumonia), higher doses and IV routes are used, guided by body weight. Renal impairment mandates dose adjustments based on creatinine clearance.
Therapeutic Indications
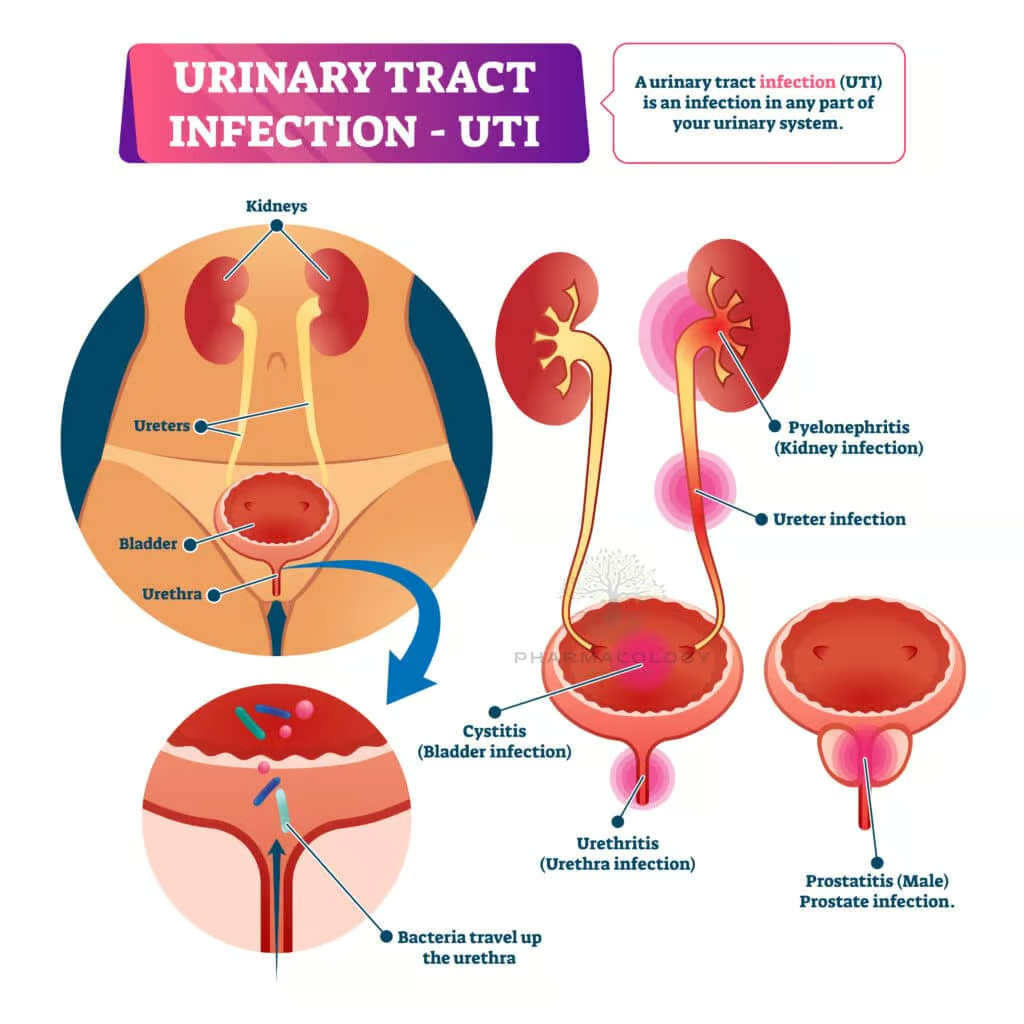
Urinary Tract Infections (UTIs)
A foundational therapy for uncomplicated UTIs, co-trimoxazole effectively eradicates E. coli and other Enterobacteriaceae, although local resistance rates require continuous surveillance. In areas with E. coli resistance levels exceeding 20%, alternative agents may be preferred (Rang & Dale, 2019).
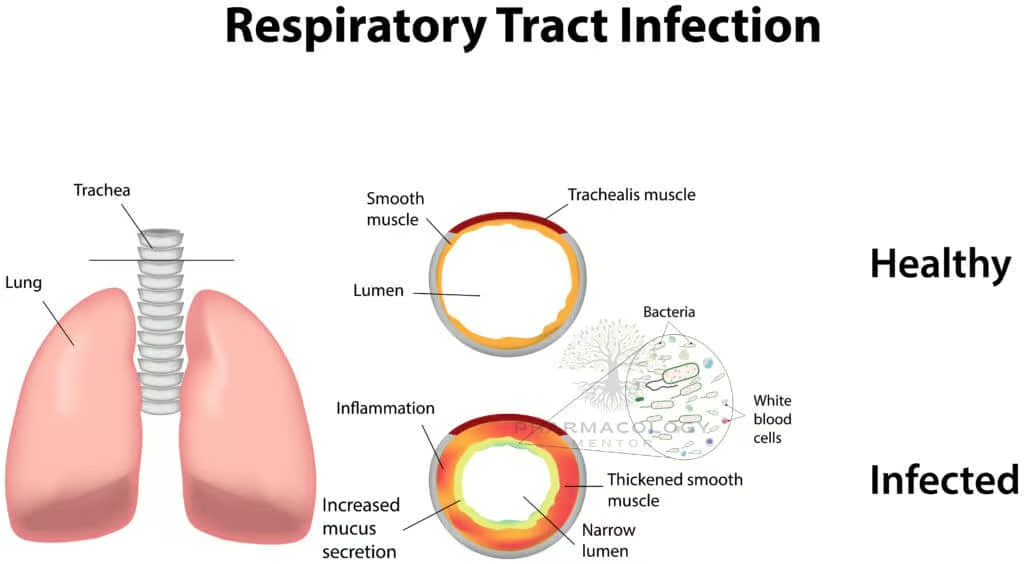
Respiratory Tract Infections
- Acute Exacerbations of Chronic Bronchitis: Co-trimoxazole can be a choice if local resistance patterns and patient allergies permit.
- Community-Acquired Pneumonia: Sometimes employed for resistant or specific etiologies.
Pneumocystis Pneumonia (PCP)
First-line for both treatment and prophylaxis in immunocompromised patients (e.g., HIV with CD4 <200 cells/µL). High-dose regimens plus adjunctive corticosteroids in severe cases significantly reduce mortality (Goodman & Gilman, 2018).
Gastrointestinal Infections
A recognized option for travelers’ diarrhea due to certain enteropathogenic E. coli, Shigella, or Salmonella. However, rising resistance in some regions necessitates alternative agents like fluoroquinolones or azithromycin (Katzung, 2020).
Other Indications
- Nocardiosis: Preferred therapy for Nocardia infections.
- Toxoplasma gondii Prophylaxis: In HIV/AIDS, co-trimoxazole prophylaxis also covers toxoplasmosis to an extent.
- MRSA Skin/Soft Tissue: In moderate outpatient infections, co-trimoxazole is frequently used if local susceptibility is verified (Rang & Dale, 2019).
Adverse Effects
Dermatological Reactions
Rashes are among the most common side effects, ranging from mild maculopapular eruptions to grave cutaneous adverse reactions like Stevens-Johnson syndrome (SJS) or toxic epidermal necrolysis (TEN). Patients with sulfonamide allergies should avoid co-trimoxazole (Goodman & Gilman, 2018).
Hematological Toxicities
- Megaloblastic Anemia: From interference with folate metabolism, predominantly in individuals with borderline folate deficiency.
- Agranulocytosis, Thrombocytopenia: Rare but can be severe.
- Hemolysis in G6PD Deficiency: Sulfonamides may induce hemolytic anemia in glucose-6-phosphate dehydrogenase-deficient patients (Katzung, 2020).
Gastrointestinal Disturbances
Nausea, vomiting, diarrhea can frequently occur. Encouraging patients to take medication with food or adequate fluids may mitigate digestive discomfort.
Hyperkalemia
Trimethoprim can reduce renal potassium excretion by modestly affecting ENaC channels, particularly in patients with renal compromise or those on other potassium-sparing agents (Rang & Dale, 2019).
Nephrotoxicity
- Crystalluria: Sulfonamides can precipitate in acidic urine, though modern sulfonamides (like sulfamethoxazole) have reduced risk. Adequate hydration is recommended.
- Acute Interstitial Nephritis: Rare immunologic phenomenon. Monitoring renal function is prudent, especially in predisposed individuals (Goodman & Gilman, 2018).
Hypersensitivity and Cross-Sensitivity
Reactions typically extend across the sulfonamide antibiotic class, but not necessarily to non-antibacterial sulfonamides (e.g., certain diuretics, sulfonylureas) since the antigenic sulfa moiety differs. Clinical caution is nonetheless advised (Katzung, 2020).
Contraindications and Precautions
- Sulfa Allergy: Absolute contraindication due to severe risk of anaphylaxis or SJS/TEN.
- Pregnancy (Late Stage): Sulfonamides risk kernicterus in neonates by displacing bilirubin. Trimethoprim’s anti-folate effect also poses teratogenic concerns, especially in the first trimester.
- G6PD Deficiency: Risk of hemolysis.
- Severe Hepatic or Renal Impairment: Necessitates dose modification or alternative treatments.
- Folate Deficiency States: Additional caution for megaloblastic anemia risk (Rang & Dale, 2019).
Drug Interactions
Oral Anticoagulants (Warfarin)
Co-trimoxazole can potentiate warfarin’s anticoagulant effects by enzyme inhibition and displacement from plasma proteins. Close INR monitoring and dosage adjustments may be required (Katzung, 2020).
Oral Hypoglycemics
Sulfonylurea metabolism and plasma protein binding can be affected by co-trimoxazole, risking hypoglycemia in diabetic patients.
ACE Inhibitors / ARBs
Concomitant use may exacerbate hyperkalemia, especially in patients with compromised renal function or on potassium-sparing regimens (Goodman & Gilman, 2018).
Methotrexate
Both sulfamethoxazole and trimethoprim can enhance methotrexate’s antifolate activity, potentially causing additive bone marrow suppression. Vigilant blood count monitoring is advised (Rang & Dale, 2019).
Resistance Mechanisms
Altered Target Enzymes
Bacterial strains may harbor mutations in dihydropteroate synthase or dihydrofolate reductase that diminish binding by sulfamethoxazole or trimethoprim, respectively.
Overproduction of Target Enzymes / Folate Pathway Components
Some organisms upregulate dihydrofolate reductase or PABA, overriding the inhibitory concentrations of co-trimoxazole.
Efflux Pumps
Active efflux systems can reduce intracellular drug concentrations.
Clinical Impact
Widespread usage for UTIs and prophylaxis fosters emerging resistant clones, particularly among E. coli. Susceptibility testing is essential to ensure efficacy. Local resistance patterns might limit co-trimoxazole’s utility in some regions (Katzung, 2020).
Special Populations
Pediatric Use
- Widely employed in older children for UTIs, otitis media, or PCP prophylaxis.
- Avoided in neonates and infants <2 months due to kernicterus risk. Dose adjustments rely on body weight, and caution is needed for G6PD deficiency (Goodman & Gilman, 2018).
Geriatric Patients
Potential for hyperkalemia, renal function decline, and medication interactions (e.g., with warfarin). Adverse skin reactions also appear more common in older adults (Rang & Dale, 2019).
HIV/AIDS Patients
Co-trimoxazole prophylaxis significantly lowers morbidity/mortality by preventing PCP, toxoplasmosis, and certain bacterial infections. Tolerance and severe hypersensitivity can be a challenge. Desensitization protocols exist for opportunistic infection prophylaxis (Katzung, 2020).
Pregnancy and Lactation
Classically avoided near term or in the first trimester. If potential benefits justify usage, monitoring for maternal-fetal complications is essential. Some guidelines do allow short courses outside critical gestational windows with appropriate caution.
Clinical Monitoring and Best Practices
- Renal Function: Baseline and periodic checks, especially in high-dose therapy (e.g., PCP) or prolonged use.
- Serum Electrolytes: Monitoring for hyperkalemia in at-risk groups.
- Blood Counts: Checking for cytopenias, especially if prolonged therapy or preexisting folate deficiency.
- Allergic Reactions: Vigilant observation for rashes or more severe cutaneous events. Quick discontinuation if significant hypersensitivity emerges (Goodman & Gilman, 2018).
Optimizing Efficacy
- Adequate Hydration: Minimizes crystalluria and renal complications.
- Awareness of MIC Data: Matching local susceptibility and breakpoint guidelines ensures appropriate application.
- Patient Counseling: Advise on photosensitivity, potential GI upset, and to discontinue promptly if rash develops (Katzung, 2020).
Comparisons and Alternatives
- Fluoroquinolones (e.g., ciprofloxacin) can substitute for UTIs or GI infections when co-trimoxazole resistance or contraindications exist, but these carry their own warnings (tendonitis, QT prolongation).
- Beta-Lactams (e.g., amoxicillin-clavulanate) may be used in respiratory or urinary pathogens with known susceptibility.
- Nitrofurantoin is an alternative for uncomplicated UTIs, especially in pregnant women after first trimester or if local E. coli remains sensitive.
- Dapsone partially parallels trimethoprim-sulfonamide anti-folate synergy in prophylaxis of Pneumocystis pneumonia but is less potent and requires additional caution for hemolysis in G6PD deficiency (Rang & Dale, 2019).
Future Directions
Overcoming Resistance
Understanding plasmid-mediated and chromosomal resistance mechanisms spurs research into novel dihydropteroate synthase and DHFR inhibitors more resilient to bacterial mutations. Certain line extensions or synergy with other antibiotic classes are being explored.
New Formulations
Investigation of long-acting or liposomal trimethoprim-sulfamethoxazole formulations for once-daily prophylaxis could improve adherence while reducing side effects. However, such developments must navigate cost-effectiveness and regulatory pathways (Katzung, 2020).
Combination Therapies
Potential synergy between co-trimoxazole and agents targeting different bacterial pathways (e.g., cell wall synthesis) may broaden coverage or reduce emerging resistance. Clinical trials must prove efficacy and safety beyond standard monotherapy or co-trimoxazole alone.
Pharmacogenomics
Variations in NAT2 or other genes may affect sulfonamide metabolism, potentially influencing toxicity risk. Pharmacogenomic screening might customize therapy—though broad clinical rollout is not yet standard (Goodman & Gilman, 2018).
Summary and Conclusions
Co-trimoxazole (the synergistic pairing of sulfamethoxazole and trimethoprim) stands as a pivotal antibacterial therapy with applications across urinary tract infections, respiratory pathogens, gastrointestinal infections, and as prophylaxis in immunocompromised patients (most notably in HIV/AIDS). Its unique mechanism rests on sequential inhibition of bacterial folate synthesis, yielding a bactericidal outcome where each agent alone might be merely bacteriostatic (Rang & Dale, 2019).
The drug’s favorable bioavailability, broad distribution, and well-established track record have sustained it amid rising concerns of antibiotic resistance. Clinically, co-trimoxazole’s roles in preventing Pneumocystis pneumonia or treating Nocardia and Toxoplasma highlight its importance in immunosuppressed populations. At the same time, increased vigilance regarding adverse effects (skin reactions, hematological toxicities, hyperkalemia), drug interactions, and contraindications (pregnancy at certain stages, sulfa allergy) remain integral to safe prescribing (Katzung, 2020).
Ultimately, co-trimoxazole persists as a cost-effective, potent antimicrobial where local resistance patterns permit. As antibiotic stewardship intensifies, prudent application—guided by organism susceptibility, patient-specific risk factors, and robust clinical monitoring—ensures that co-trimoxazole continues to serve as a critical defense in the anti-infective arsenal. Ongoing research into resistance mechanisms, evolving doping strategies (e.g., new formulations, synergy approaches), and targeted prophylaxis regimens may expand or maintain co-trimoxazole’s standing in modern pharmacotherapy (Goodman & Gilman, 2018).
References (Book Citations)
- Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 13th Edition.
- Katzung BG, Basic & Clinical Pharmacology, 14th Edition.
- Rang HP, Dale MM, Rang & Dale’s Pharmacology, 8th Edition.









