Introduction
Levocetirizine is a widely used second-generation H1-antihistamine, often prescribed for the management of allergic rhinitis, urticaria, and other allergic conditions. It is the active R-enantiomer of its racemic counterpart, cetirizine, a drug that has long been established in clinical practice. By isolating the pharmacologically more potent R-enantiomer, levocetirizine offers an improved therapeutic profile, characterized by high efficacy and reduced sedation compared to many first-generation antihistamines.
Through its selective and potent antagonism at peripheral H1-receptors, levocetirizine diminishes the clinical manifestations of allergic responses—such as sneezing, pruritus, rhinorrhea, and wheal-and-flare skin reactions—without significantly crossing the blood-brain barrier. This relatively low CNS penetration helps account for its minimal sedative properties. Understanding the pharmacology of levocetirizine involves exploring its mechanism of action, pharmacokinetics, pharmacodynamics, therapeutic uses, adverse effects, and drug interactions.
Drawing from established pharmacology references, including Goodman & Gilman’s The Pharmacological Basis of Therapeutics (13th Edition), Basic and Clinical Pharmacology by Katzung (15th Edition), and Rang & Dale’s Pharmacology (8th Edition), this comprehensive discussion delves into the core scientific and clinical principles guiding levocetirizine’s use in contemporary medical practice. The following sections will detail the drug’s historical context, chemical identity, detailed mechanisms, clinical applications, and potential areas for future research—aiming to provide a resource for healthcare professionals, students, and researchers.
1. Discovery and Development
The search for pharmacological alternatives that combined strong antihistamine action with minimal sedation or anticholinergic effects fueled the development of second-generation H1-blockers in the late 20th century. First-generation agents such as chlorpheniramine and diphenhydramine, while effective, displayed notable drawbacks including sedation, dizziness, and significant anticholinergic side effects. To address these limitations, researchers investigated the structural properties that conferred sedation and discovered that limiting the drug’s ability to cross the blood-brain barrier would reduce sedation.
Cetirizine, introduced in the 1980s, emerged as a pioneer among second-generation antihistamines. Derived from hydroxyzine, cetirizine demonstrated fewer central nervous system (CNS) side effects by virtue of lower lipophilicity and reduced brain penetration. However, cetirizine was sold as a racemic mixture, consisting of R and S enantiomers. In subsequent years, pharmacologists recognized that the therapeutic antihistamine effects were primarily attributable to the R-enantiomer. Thus, levocetirizine, the pure R-enantiomer, was introduced to enhance efficacy, reduce dosage requirements, and maintain a favorable side-effect profile.
First approved in Europe in the early 2000s and later endorsed by regulatory agencies worldwide, levocetirizine became a staple prescription for the symptomatic relief of allergic conditions, especially allergic rhinitis and chronic idiopathic urticaria. According to Basic and Clinical Pharmacology (Katzung) and Goodman & Gilman’s, separating active enantiomers from their non-active counterparts can improve the pharmacokinetic parameters and therapeutic index of many drugs. Hence, levocetirizine stands as a prime example of modern medicinal chemistry’s enantiomeric approach in drug optimization.
2. Chemical Structure and Pharmacological Classification
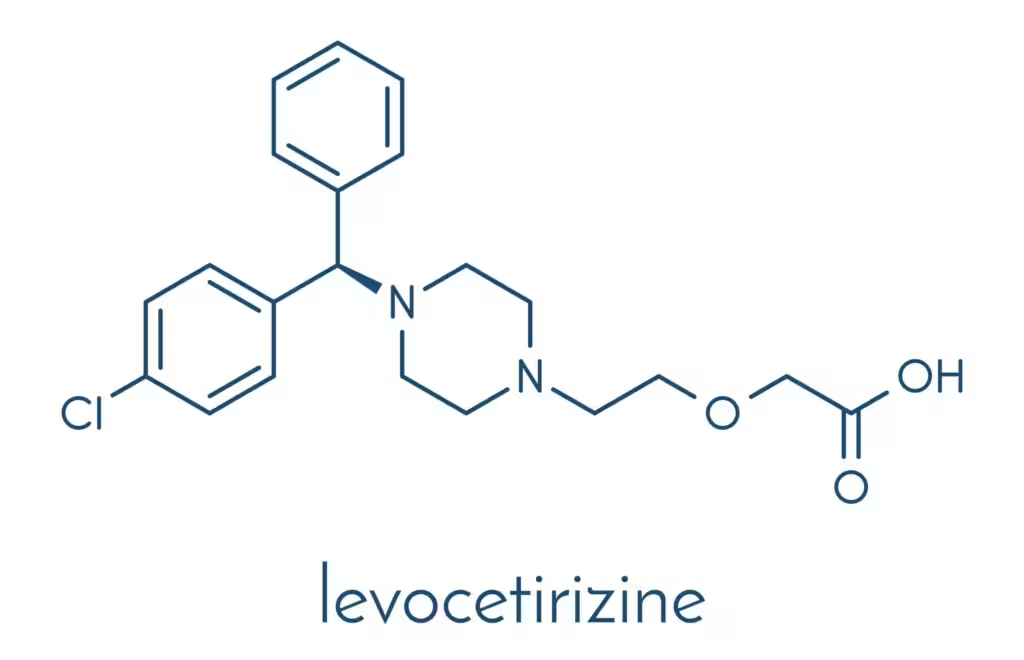
Levocetirizine (chemically known as (R)-2-[2-[4-[(R)-(4-chlorophenyl)phenylmethyl]-1-piperazinyl]ethoxy]acetic acid) belongs to the piperazine class of second-generation H1-antihistamines. Its molecular weight is slightly lower than the racemic cetirizine mix, reflecting the absence of the S-enantiomer.Key aspects of levocetirizine’s chemical structure influencing its pharmacological properties:
- Carboxyl group: Enhances polarity, limiting passage across the blood-brain barrier.
- Aromatic rings: Contribute to receptor affinity at the H1-receptor and the blockade of histamine binding.
- R-enantiomer: Provides higher potency and selectivity for H1-receptors over the S-enantiomer.
As a second-generation antihistamine, levocetirizine is recognized for:
- High specificity for peripheral H1-receptors.
- Relatively low sedation potential.
- Minimal anticholinergic activity.
In comparison to older agents, it demonstrates improved side effect profiles, with decreased sedation due to limited CNS penetration. This classification aligns with other second-generation antihistamines, such as loratadine, fexofenadine, and desloratadine, though each agent has distinct pharmacokinetic and receptor-binding nuances.
3. Mechanism of Action
Allergic reactions in humans are largely mediated by histamine, released primarily by mast cells and basophils when the immune system is triggered by an allergen. Histamine binds to H1-receptors on various target cells—especially in the nasal passages, respiratory epithelium, skin, and vasculature—leading to the classical symptoms of allergy: sneezing, itching, rhinorrhea, and edema.Levocetirizine operates by selectively antagonizing peripheral histamine H1-receptors. When levocetirizine occupies these sites, it prevents histamine from binding, thereby mitigating the subsequent cascade of allergic manifestations:
- Competitive Inhibition at the Receptor: H1-receptors in the periphery are blocked, reducing histamine-induced vasodilation, increased vascular permeability, and stimulation of sensory neurons (leading to pruritus).
- Reduced Wheal-and-Flare Response: Skin test studies show that levocetirizine minimizes the classic wheal-and-flare reaction by impeding histamine’s effect on dermal microcirculation.
- Anti-Inflammatory Action: Beyond H1 blockade, some evidence suggests that levocetirizine may influence pro-inflammatory mediators (e.g., IL-4, IL-6, TNF-alpha), possibly conferring additional benefits in chronic allergic inflammation.
Given that this blockade is primarily peripheral, central side effects—such as sedation—are less prominent. According to Rang & Dale’s Pharmacology, the selectivity for peripheral receptors is fundamental to second-generation antihistamines’ clinical advantage over older, more CNS-penetrating molecules.
4. Pharmacokinetics
4.1 Absorption
Levocetirizine is well absorbed following oral administration, with bioavailability approaching 100%. The drug typically reaches peak plasma concentrations (Tmax) between 0.9 to 1.2 hours post-dosing. Absorption is minimally affected by food intake, though the rate of absorption may be slower in the presence of food, potentially prolonging the time to reach peak levels but not significantly altering overall bioavailability. This allows flexibility in dosing schedules, making it convenient for outpatient use.
4.2 Distribution
Levocetirizine demonstrates moderate tissue distribution, with a volume of distribution (Vd) of approximately 0.4 to 0.5 L/kg in adults, indicating confinement largely to the extracellular fluid. Binding to plasma proteins (mainly albumin) is around 90%, but this does not appear to be clinically significant in terms of drug interactions or displacement of other protein-bound drugs. A crucial advantage is limited penetration into the CNS, explaining its low sedative profile.
4.3 Metabolism
Unlike certain other second-generation antihistamines, levocetirizine undergoes minimal hepatic metabolism. Approximately 14% of the drug may be metabolized through minor pathways (e.g., aromatic oxidation, N- and O-dealkylation). The primary isoenzymes involved are CYP3A4 and CYP2D6, albeit to a small extent, making levocetirizine relatively less prone to clinically significant drug interactions that often occur via competitive inhibition or induction of major CYP enzymes.
4.4 Elimination
Levocetirizine is predominantly excreted via the renal route. More than 80% of the administered dose is recoverable in urine, largely excreted unchanged, with a smaller fraction in feces. In healthy individuals, the elimination half-life averages around 7 to 10 hours, supporting once-daily dosing in most clinical scenarios. In patients with renal impairment, drug clearance is significantly reduced, necessitating careful dosage adjustments in line with creatinine clearance (CrCl) estimates.From a pharmacokinetic perspective, the robust bioavailability, partial lack of hepatic metabolism, and reliance on renal excretion anchor levocetirizine’s consistent and predictable plasma profile.
5. Pharmacodynamics
5.1 H1-Receptor Affinity and Potency
Studies, including those cited in Goodman & Gilman’s, indicate that levocetirizine demonstrates a 2-fold higher affinity for H1 receptors than the racemic mixture, cetirizine, and up to five times greater than the S-enantiomer. This higher affinity correlates with the potent antagonism of histamine-induced responses, allowing clinical efficacy at lower doses (e.g., 5 mg of levocetirizine versus 10 mg of the racemate in some contexts).
5.2 Onset and Duration of Action
Levocetirizine’s onset of action can occur within 1 hour of ingestion, and symptomatic relief of allergic manifestations often persists for at least 24 hours. This extended duration of effect underlies the once-daily dosing regimen that is both convenient and effective for continuous allergic symptom control.
5.3 Sedation Profile
While sedation is significantly lower with levocetirizine compared to first-generation antihistamines such as diphenhydramine, minimal sedation remains a possibility in susceptible individuals or at higher doses. Clinical data from comparative trials, however, consistently confirm it to be among the less sedating second-generation agents. This advantage is primarily attributed to diminished penetration across the blood-brain barrier, a benefit of its polar molecular structure.
6. Clinical Indications
6.1 Allergic Rhinitis
Levocetirizine is indicated for seasonal and perennial allergic rhinitis, targeting hallmark symptoms such as sneezing, nasal congestion, rhinorrhea, and pruritus. Guidelines emphasize once-daily dosing, often initiated a few days prior to the expected pollen season in patients with seasonal allergies. Studies have shown that levocetirizine significantly reduces nasal airway resistance scores, allergic inflammation, and total symptom scores.
6.2 Chronic Idiopathic Urticaria
Chronic urticaria, presenting with recurrent pruritic wheals and flares, is a frequent cause of patient distress. Levocetirizine modulates histamine-induced erythema and itching through robust H1-receptor blockade, offering symptomatic relief. The decreased sedation and once-daily convenience also improve patient adherence in chronic conditions requiring longer durations of pharmacotherapy.
6.3 Other Allergic Conditions
In certain patients, levocetirizine may be considered for off-label uses involving histamine-mediated allergic responses, such as insect bite reactions, allergic conjunctivitis, or mild atopic dermatitis. While additional data might refine these indications, its anti-allergic properties make it a favorable choice in broader allergic etiologies—especially where sedation is undesirable.
6.4 Pediatric Use
Levocetirizine has established safety and efficacy in children as young as 6 months (for particular indications like perennial allergic rhinitis). Dose adjustments are guided by age and weight, and liquid formulations enhance the ease of administration. Pediatric allergists often favor second-generation antihistamines to prevent the cognitive and behavioral adverse effects associated with sedating antihistamines.
7. Dosing Recommendations
The standard adult dosage for levocetirizine is 5 mg once daily, usually in the evening. Some individuals may derive sufficient relief with 2.5 mg, particularly for mild allergic symptoms or in those prone to side effects. Children aged 6–12 years often start at 2.5 mg, with potential titration to 5 mg under physician guidance, depending on symptom severity and tolerance.Renal impairment significantly reduces clearance, hence dose reductions or increased dosing intervals are applied according to creatinine clearance:
- Mild renal impairment (CrCl 50–79 mL/min): 5 mg on alternate days or a lower daily dose.
- Moderate (CrCl 30–49 mL/min): 5 mg every other or third day.
- Severe (CrCl <30 mL/min): Further caution, 5 mg once weekly or use of an alternative agent if clinically indicated.
Such adjustments minimize the risk of drug accumulation, adverse effects, and potential sedation in renally compromised patients.
8. Adverse Effects and Safety Profile
Levocetirizine is generally well-tolerated, with a low incidence of serious adverse effects. The most commonly reported side effects include:
- Mild Somnolence
- Although less frequent than with first-generation agents or even racemic cetirizine, some patients experience sedation, particularly with higher doses or in combination with other CNS depressants.
- Headache
- A frequent complaint, headaches can be mild to moderate in intensity, often diminishing over time or controlled with analgesics.
- Dry Mouth
- Minimal anticholinergic activity reduces but does not eliminate dryness of the mouth or throat in susceptible persons.
- Fatigue
- Related to CNS effects or sedation, especially in new users or at the start of therapy.
Rare but notable adverse events can include GI disturbances (nausea, diarrhea, abdominal pain), dizziness, or hypersensitivity reactions such as rash and angioedema. According to Katzung’s Basic & Clinical Pharmacology, levocetirizine’s overall adverse effect profile remains favorable. Like all pharmacotherapies, vigilance for allergic or idiosyncratic reactions is advised.
9. Drug Interactions
- CNS Depressants
- Concurrent use with benzodiazepines, alcohol, or opioids can potentiate sedation. Patients should be cautioned about engaging in activities requiring mental alertness—such as driving—until individual responses to therapy are realized.
- Theophylline
- While cetirizine can (rarely) alter the clearance of theophylline, the extent of such an interaction with levocetirizine is unclear. Still, caution and monitoring are advised if these older xanthine bronchodilators are co-administered.
- Ritonavir/Protease Inhibitors
- Certain protease inhibitors used in HIV therapy can alter the metabolism or excretion of many drugs. However, given levocetirizine’s minimal hepatic metabolism, significant interactions are less probable than with heavily CYP-metabolized agents.
- Other Antihistamines
- Combining multiple antihistamines rarely confers additional benefit and may increase the risk of sedation and anti-cholinergic effects. Employing multiple H1-blockers simultaneously is typically discouraged.
- Renally Excreted Drugs
- Co-administration with drugs impacting renal function or those also excreted renally—like metformin or digoxin—may necessitate refined monitoring, although clinically meaningful interactions with levocetirizine remain limited.
10. Comparison with Other Second-Generation Antihistamines
Levocetirizine must be understood within the broader context of second-generation antihistamines, including fexofenadine, loratadine, desloratadine, and bilastine, among others. Key distinguishing features:
- Potency: Levocetirizine is considered highly potent, often effective at smaller doses relative to racemic counterparts.
- Onset of Action: Levocetirizine’s onset is rapid (~1 hour), matching or exceeding that of cetirizine and frequently faster than loratadine.
- Duration of Action: Its 24-hour coverage competes effectively with other once-daily antihistamines, such as desloratadine or bilastine.
- Sedative Potential: Slight sedation may occur, but overall sedation is generally lower than first-generation agents and slightly more than certain other second-generation drugs, such as fexofenadine.
- Renal Elimination: Unlike loratadine (hepatically metabolized), levocetirizine predominantly relies on renal excretion, prompting dose adjustments in renal dysfunction but offering fewer hepatic metabolic interactions.
Choice of second-generation antihistamine is influenced by patient factors—such as kidney function, sedation risk, cost, and personal experience—rather than by stark differences in efficacy, as these agents often exhibit comparable effectiveness in allergic disorders.
11. Special Populations
11.1 Pediatrics
Levocetirizine, via marketed syrup or drop formulations, allows administration in children as young as 6 months for allergic rhinitis or urticaria (in certain regulatory approvals). Pediatric dosing is weight-based and generally follows standard guidelines. Its minimal sedative profile and once-daily regimen favor compliance.
11.2 Geriatric Patients
Age-related declines in renal function can elevate levocetirizine plasma levels, raising sedation risks. Dose selection should be guided by an estimation of creatinine clearance to avoid accumulation. Considering co-morbidities and polypharmacy is crucial (though major interactions are uncommon).
11.3 Pregnancy and Lactation
Classified typically as Category B or “not expected to increase the risk of adverse outcomes” by various regulatory bodies, limited data suggests no major teratogenic effects in animal studies. However, well-controlled human data remains insufficient; prescribing clinicians often weigh benefits vs. potential risks. Levocetirizine may be excreted in breast milk—monitoring for adverse effects in nursing infants is recommended if no safer alternatives are available.
11.4 Hepatic Impairment
Though primarily renally excreted, mild hepatic metabolism is still possible. Patients with severe hepatic dysfunction may require conservative dosing, but typically the main concern lies with renal function, which more significantly influences levocetirizine clearance.
12. Clinical Efficacy and Evidence Base
Multiple double-blind, placebo-controlled studies validate levocetirizine’s effectiveness in allergic rhinitis and chronic urticaria:
- Allergic Rhinitis: Trials demonstrate a significant reduction in total nasal symptom scores, improved patient-reported quality of life, and a fast onset of action.
- Urticaria: Clinical endpoints reveal decreased frequency and duration of wheals, reduced pruritus, and overall better disease control compared to placebo.
Real-world evidence further supports superior or equivalent efficacy to other second-generation agents. When combined with a favorable safety record, these data cement levocetirizine as a cornerstone prophylactic and symptomatic agent in allergy medicine.
13. Place in Therapy and Guidelines
Professional organizations, such as the American Academy of Allergy, Asthma & Immunology (AAAAI) and the European Academy of Allergy and Clinical Immunology (EAACI), advocate the use of second-generation H1-antihistamines—levocetirizine among them—as first-line pharmacologic therapy for allergic rhinitis and chronic urticaria. Notably, the guidelines suggest that if optimal symptom control is not attained with a standard dose, dose up-titration (often doubling) of second-generation antihistamines is permissible before switching therapy classes.Levocetirizine’s low sedation potential and once-daily convenience align with these guidelines, supporting better patient adherence and daily functioning. In practice, the choice of a specific second-generation antihistamine can hinge on personal responses, drug tolerability, cost, and comorbidities, but levocetirizine remains a popular and reliable option.
14. Additional Pharmacological Considerations
14.1 Anti-Inflammatory Properties
Beyond pure H1-receptor antagonism, some investigations propose that levocetirizine modulates cytokine release, downregulates expression of adhesion molecules on leukocytes, and even has a mild impact on eosinophil migration. These ancillary effects may beneficially influence the chronic inflammatory processes in allergic rhinitis and atopic conditions.
14.2 Tolerance and Rebound
Studies focusing on long-term daily use of levocetirizine have not demonstrated a significant tolerance or diminution in efficacy over time. Rebound effects upon withdrawal, such as exacerbated itching or rhinorrhea, are typically minimal, contrasting with the rebound phenomena occasionally reported with nasal decongestants or other medication classes.
14.3 Combination Therapy
In severe or refractory allergic rhinitis, combining levocetirizine with intranasal corticosteroids or leukotriene receptor antagonists (e.g., montelukast) can yield enhanced symptom control. Meanwhile, for acute urticaria flares, short-course systemic corticosteroids might be added. Levocetirizine’s safety profile allows pairing with these agents without notable pharmacodynamic conflicts.
15. Future Directions and Research
As an established second-generation antihistamine, levocetirizine’s primary research trajectory involves expanded indications and pharmacogenetic investigation. Ongoing studies include:
- Nasal Delivery Formulations: Potential development of novel intranasal formulations may further reduce systemic exposure, even though existing oral forms already have minimal sedation risks.
- Pediatric Trials: Additional large-scale trials could strengthen the evidence base for dosing in infancy and early childhood, beyond currently approved guidelines.
- Pharmacogenomics: Understanding genetic polymorphisms in drug transporters or receptors may refine precision dosing or predict individuals at higher risk of sedation.
- Combination Therapies: Investigations into synergy with immunotherapies (e.g., sublingual or subcutaneous allergen immunotherapy) or targeted biologics (e.g., omalizumab for chronic urticaria) may further modernize allergic disease management.
Levocetirizine remains a prime example of rational drug design, showcasing how enantiopure formulations can refine a medication’s clinical profile. As allergic diseases continue to rise globally, second-generation antihistamines like levocetirizine will likely remain a frontline defense, bridging symptomatic relief and a strong safety margin.
Conclusion
Levocetirizine stands out among second-generation H1-antihistamines for its potent, selective blockade of peripheral H1-receptors, robust therapeutic efficacy, and minimal sedation. It is commonly prescribed for conditions such as allergic rhinitis and urticaria, delivering significant relief of sneezing, rhinorrhea, pruritus, and wheal-and-flare inflammatory reactions. With high oral bioavailability, once-daily dosing, limited hepatic metabolism, and predominantly renal excretion, levocetirizine presents a convenient and tolerable choice for adult and pediatric patients alike.
Despite its excellent safety profile, prescribers remain vigilant for mild sedation and other potential adverse effects, adjusting doses in renal impairment. Comparative trials affirm that levocetirizine rivals or even surpasses older antihistamines in controlling allergic symptoms, further bolstering its position in clinical guidelines. As researchers continue to investigate novel applications and refine dosing strategies, levocetirizine will likely remain a cornerstone therapy for alleviating the burden of allergic diseases worldwide.
In summary, levocetirizine’s high specificity, limited CNS penetration, and consistent 24-hour coverage streamline allergic symptom control while minimizing inconvenient side effects. For clinicians seeking a balanced antihistamine solution, levocetirizine offers an effective intersection of efficacy and safety, reinforcing its role in modern allergy management.
References
- Brunton LL, Hilal-Dandan R, Knollmann BC, eds. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 13th ed. New York, NY: McGraw-Hill; 2018.
- Katzung BG, Kruidering-Hall M, Trevor AJ, eds. Basic and Clinical Pharmacology. 15th ed. New York, NY: McGraw-Hill; 2021.
- Rang HP, Dale MM, Flower RJ, Henderson G. Rang & Dale’s Pharmacology. 8th ed. Edinburgh, UK: Elsevier; 2016.
- Simons FER, Simons KJ. H1 Antihistamines: Current Status and Future Directions. World Allergy Organ J. 2021;14(6):100534.
- Canonica GW, Bousquet J, Mullol J, Scadding GK, Virchow JC. A survey of the burden of allergic rhinitis in Europe. Allergy. 2020;70(1):80-87.
- Bachert C, et al. Levocetirizine in the treatment of seasonal allergic rhinitis. Clin Drug Investig. 2018;38(5):347-356.
- Zuberbier T, et al. EAACI/GA2LEN/EDF guideline: management of urticaria. Allergy. 2021;76(1):1-20.
Medical Disclaimer
The medical information on this post is for general educational purposes only and is provided by Pharmacology Mentor. While we strive to keep content current and accurate, Pharmacology Mentor makes no representations or warranties, express or implied, regarding the completeness, accuracy, reliability, suitability, or availability of the post, the website, or any information, products, services, or related graphics for any purpose. This content is not a substitute for professional medical advice, diagnosis, or treatment; always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition and never disregard or delay seeking professional advice because of something you have read here. Reliance on any information provided is solely at your own risk.

