Introduction
Anticoagulants constitute an essential class of drugs used to inhibit blood coagulation, ultimately preventing or treating thromboembolic disorders such as deep vein thrombosis (DVT), pulmonary embolism (PE), and stroke in atrial fibrillation (AF). By targeting specific elements in the clotting cascade—whether upstream or downstream—anticoagulants forestall the formation, propagation, or recurrence of harmful clots. However, because these agents tamper with the delicate equilibrium between bleeding and clotting, clinicians must carefully weigh their benefits against hemorrhagic risks.
This article provides a comprehensive, up-to-date overview of the pharmacology of anticoagulants, referencing standard pharmacology textbooks (Goodman & Gilman’s, Katzung, and Rang & Dale’s). We’ll discuss the mechanisms, pharmacokinetics, clinical uses, and adverse effects of both traditional anticoagulants (such as Heparin, Warfarin) and newer oral anticoagulants (NOACs/DOACs)—spanning Direct Factor Xa Inhibitors (e.g., Rivaroxaban, Apixaban) and Direct Thrombin Inhibitors (e.g., Dabigatran). By understanding how these agents act, and how they may be monitored or reversed, clinicians can optimize patient outcomes and minimize complications.
Hemostasis and the Rationale for Anticoagulation
Overview of Hemostatic Pathways
The hemostatic mechanism is typically divided into two pathways—primary hemostasis (platelet plug formation) and secondary hemostasis (coagulation cascade). In secondary hemostasis, clotting factors sequentially activate one another, culminating in the generation of thrombin, which converts fibrinogen into fibrin. Fibrin strands then reinforce the platelet plug. The main arms of the coagulation cascade:
- Intrinsic Pathway (factors XII, XI, IX, VIII)
- Extrinsic Pathway (tissue factor/factor VII)
- Common Pathway (factors X, V, II [prothrombin], and I [fibrinogen])
Thrombin (factor IIa) also exerts positive feedback on platelets and earlier clotting factors, promoting robust clot development. Anticoagulants govern clotting by:
- Inhibiting key clotting factors (e.g., Heparin augmenting antithrombin III or direct factor Xa/thrombin inhibitors).
- Interfering with vitamin K–dependent factor synthesis (e.g., Warfarin).
Pathophysiology of Thrombosis
Excessive or inappropriate clot formation (thrombosis) can arise from Virchow’s triad: endothelial injury, stasis or turbulent blood flow, and hypercoagulability. When these factors disrupt hemostatic balance, venous thrombi may block lung circulation (PE), and arterial thrombi may cause myocardial infarction or stroke. Anticoagulation reduces these events by limiting fibrin generation, essential for stable clot formation.
Classification of Anticoagulants
1. Parenteral Anticoagulants
- Unfractionated Heparin (UFH)
- Low–Molecular-Weight Heparins (LMWHs): Enoxaparin, Dalteparin
- Fondaparinux
- Direct Thrombin Inhibitors (Parenteral): Bivalirudin, Argatroban (and Lepirudin historically)
2. Oral Vitamin K Antagonists
- Warfarin (Coumadin)
3. Direct Oral Anticoagulants (DOACs)
- Direct Factor Xa Inhibitors: Rivaroxaban, Apixaban, Edoxaban, Betrixaban
- Direct Thrombin Inhibitor: Dabigatran etexilate
Each class offers distinct mechanisms, routes, half-lives, and monitoring requirements, shaping their role in clinical settings such as venous thromboembolism prophylaxis, stroke prevention in AF, and acute coronary syndromes.
Parenteral Anticoagulants
Unfractionated Heparin (UFH)
Chemical and Biological Properties
Heparin is a heterogeneous mixture of sulfated glycosaminoglycan chains, primarily extracted from porcine intestinal mucosa or bovine sources. Its molecular weight ranges widely (3,000–30,000 Da).
Mechanism of Action
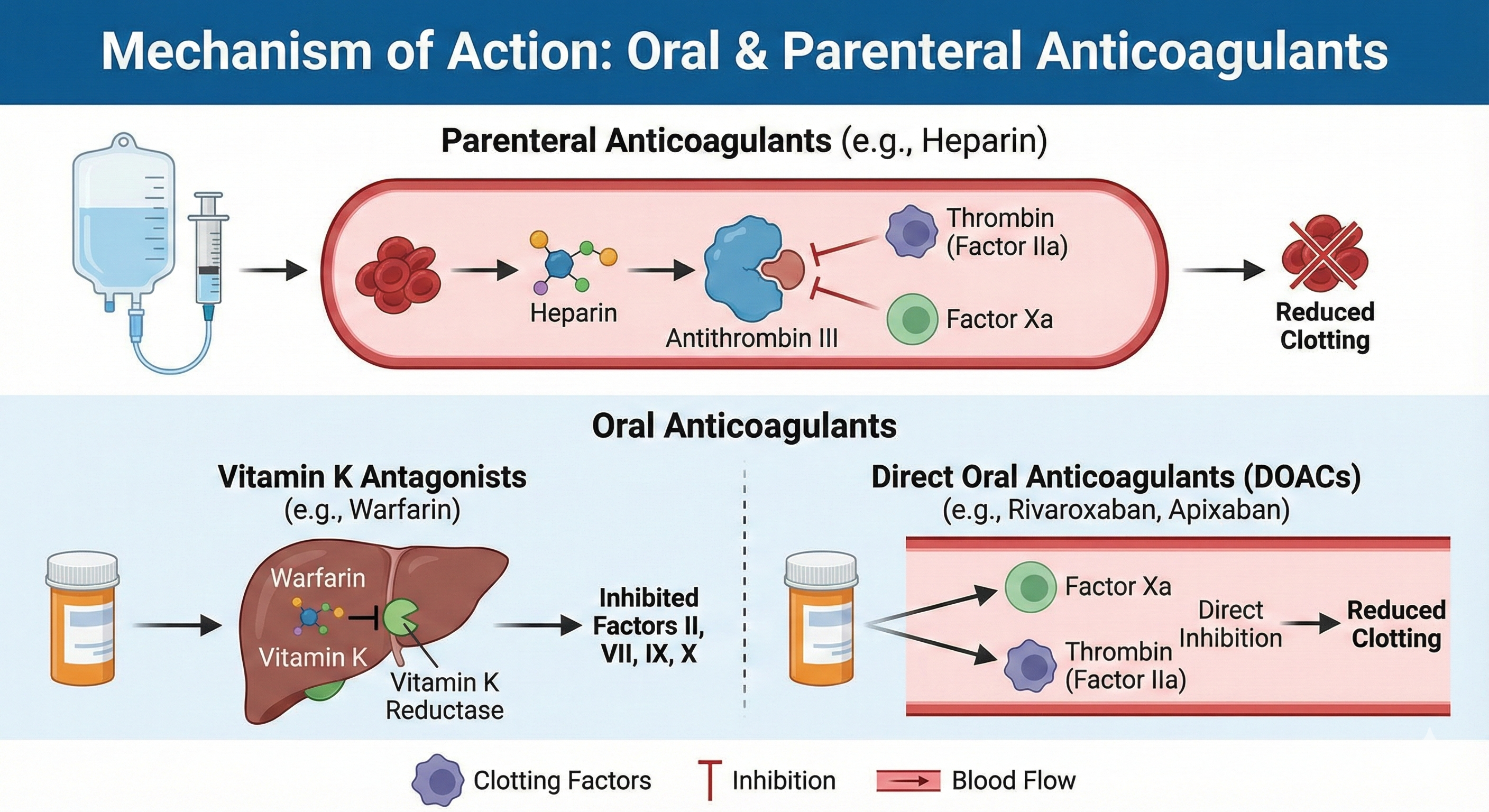
Heparin exerts its anticoagulant effect by binding to antithrombin III (ATIII), inducing a conformational change that accelerates the inactivation of clotting factors—particularly factor Xa and thrombin (factor IIa). For robust thrombin inhibition, a longer heparin chain (at least 18 saccharide units) is needed to bridge antithrombin and thrombin. Consequently, UFH can inhibit both factor Xa and IIa effectively.
Pharmacokinetics
- Administration: IV infusion or subcutaneous injection.
- Onset of Action: Immediate with IV; ~1–2 hours subcutaneously.
- Monitoring: Activated Partial Thromboplastin Time (aPTT) or anti-Xa levels used to guide dosing.
- Half-Life: ~1–2 hours, but can be longer at higher doses.
- Metabolism: Heparin is cleared hepatically and also by the reticuloendothelial system.
Clinical Uses
- Treatment and prophylaxis of DVT, PE.
- Acute coronary syndromes (unstable angina, myocardial infarction).
- Cardiopulmonary bypass, vascular or cardiac surgery.
- Bridging therapy while initiating warfarin.
Adverse Effects
- Bleeding: The major complication, requiring close monitoring.
- Heparin-Induced Thrombocytopenia (HIT): An immune-mediated prothrombotic disorder characterized by platelet factor 4 (PF4) antibodies.
- Osteoporosis: Long-term use.
- Hypersensitivity (rare).
Reversal
- Protamine Sulfate: Binds negatively charged heparin, neutralizing its effect. Partial reversal for LMWH but not for fondaparinux.
Low–Molecular-Weight Heparins (LMWHs)
Examples include Enoxaparin and Dalteparin. Produced by chemical or enzymatic depolymerization of UFH, they mainly target factor Xa (less so thrombin) due to shorter chain lengths.
Mechanism of Action
LMWHs bind antithrombin, preferentially inactivating factor Xa. They exhibit reduced bridging capacity to inhibit thrombin effectively.
Pharmacokinetics
- Subcutaneous administration, near-complete bioavailability.
- Longer half-life (~2–4 hours) than UFH.
- Monitoring: Generally not necessary for routine prophylaxis; can measure anti–factor Xa in special populations (renal impairment, extreme body weight, pregnancy).
- Renal Excretion: Accumulation concerns in significant renal dysfunction.
Clinical Uses
- Prevention and treatment of DVT/PE (inpatient and outpatient settings).
- Bridging therapy while starting warfarin in high-risk patients.
- Acute coronary syndromes (e.g., unstable angina).
Side Effects
- Bleeding, albeit reduced risk compared to UFH.
- HIT risk is lower vs. UFH but still possible.
- Injection-site reactions.
Fondaparinux
A synthetic pentasaccharide that selectively binds antithrombin, inhibiting factor Xa without direct impact on thrombin.
Pharmacokinetics
- Subcutaneous route, with nearly 100% bioavailability.
- Once-daily dosing due to longer half-life (~17–21 hours).
- Renal Excretion: Contraindicated if CrCl <30 mL/min.
Uses and Adverse Reactions
- VTE prophylaxis (orthopedic surgery).
- Treatment of DVT, PE (often combined with a vitamin K antagonist until stable).
- Bleeding is a risk, no direct reversal agent, though prothrombin complex concentrates (PCC) may help in severe hemorrhage.
- HIT is extremely rare with fondaparinux.
Parenteral Direct Thrombin Inhibitors
Include Bivalirudin, Argatroban, and historically Lepirudin. They directly bind thrombin’s active site, preventing cleavage of fibrinogen.
Clinical Application
- Bivalirudin: Primarily used during percutaneous coronary interventions (PCI) in patients with HIT or at high risk.
- Argatroban: Preferred in HIT with or without thrombosis.
- Monitoring: aPTT or direct assays for some agents.
- Short Half-Lives: Typically IV infusion with rapid onset/offset.
Oral Vitamin K Antagonists
Warfarin
Mechanism of Action
Warfarin interferes with the hepatic synthesis of vitamin K–dependent clotting factors (II, VII, IX, X) and proteins C and S, by inhibiting the enzyme vitamin K epoxide reductase complex 1 (VKORC1). Without reduced vitamin K, gamma-carboxylation of these factors is impaired, rendering them functionally deficient.
Pharmacokinetics
- Oral administration, nearly complete absorption.
- Highly protein-bound, leading to potential drug interactions.
- Long half-life of ~40 hours, though full therapeutic effect requires depletion of existing clotting factors over several days.
- Metabolized by CYP2C9, susceptible to numerous drug or dietary interactions (e.g., leafy vegetables high in vitamin K).
Monitoring
- Prothrombin Time (PT), reported as International Normalized Ratio (INR). Typical therapeutic range is an INR of 2.0–3.0 for most indications, or 2.5–3.5 for mechanical heart valves in certain positions.
Clinical Indications
- Long-term prophylaxis/treatment of DVT, PE.
- Stroke prevention in atrial fibrillation.
- Mechanical heart valve anticoagulation.
Adverse Effects
- Bleeding: Intracranial hemorrhage, GI bleeds, etc.
- Teratogenicity: Crosses the placenta; not recommended in pregnancy (especially first trimester).
- Skin necrosis: Rare, related to protein C deficiency early in therapy.
- Purple toe syndrome: Rare cholesterol microembolization phenomenon.
Reversal Agents
- Vitamin K1 (phytonadione): Oral or IV for elevated INR/bleeding.
- Prothrombin Complex Concentrate (PCC): For urgent correction in severe bleeding.
- Fresh Frozen Plasma (FFP): Alternative but less concentrated in clotting factors.
Direct Oral Anticoagulants (DOACs)
Direct Factor Xa Inhibitors
These agents selectively and reversibly block factor Xa in its free and clot-bound forms, reducing thrombin generation.
Rivaroxaban
- Mechanism: Directly inhibits factor Xa without antithrombin.
- Pharmacokinetics: Oral, once or twice daily (dose-dependent), partial renal excretion.
- Indications: Stroke prevention in AF, DVT/PE treatment and prophylaxis, post-orthopedic surgery prophylaxis.
- Monitoring: Routine lab tests usually unnecessary; anti-factor Xa assay can be used in special circumstances.
- Adverse Effects: Bleeding (particularly GI), potential drug interactions (CYP3A4, P-gp).
Apixaban
- Similar to rivaroxaban but often has a low bleeding risk—especially GI bleeds—and is dosed twice daily.
- Approved for non-valvular AF stroke prevention and VTE therapy.
- Less renal excretion than rivaroxaban, but still some caution in significant renal impairment.
Edoxaban
- Once-daily factor Xa inhibitor.
- Approved in AF and VTE treatment.
- Not recommended if creatinine clearance is very high (>95 mL/min) due to possibly reduced efficacy in preventing stroke in AF.
Betrixaban
- Primarily indicated for extended prophylaxis of VTE in acutely ill hospitalized patients.
- Once daily, with minimal renal elimination.
- Observed to reduce VTE risk over longer durations.
Direct Thrombin Inhibitor: Dabigatran Etexilate
- Prodrug converted to dabigatran, which directly binds thrombin (factor IIa).
- Indicated for stroke prevention in non-valvular AF, DVT/PE treatment, and secondary prevention.
- Renal clearance is significant, so adjustment needed in reduced kidney function.
- Adverse effects: GI bleeding, dyspepsia.
DOAC Adverse Effects and Reversal
- Bleeding remains the primary concern.
- Idarucizumab specifically reverses dabigatran.
- Andexanet alfa used for factor Xa inhibitor reversal (e.g., rivaroxaban, apixaban).
- Prothrombin complex concentrates (PCC) or activated PCC sometimes utilized off-label for urgent reversal.
Comparative Aspects of DOACs vs. Warfarin

- Onset: DOACs achieve therapeutic levels within hours, whereas warfarin takes days.
- Monitoring: DOACs generally do not require routine INR checks. Warfarin necessitates frequent INR monitoring to maintain therapeutic range.
- Dietary Interactions: DOACs are not influenced by vitamin K intake, whereas warfarin’s effects can fluctuate with dietary vitamin K.
- Drug Interactions: DOACs have fewer interactions but remain sensitive to P-gp and/or CYP3A4 inducers or inhibitors. Warfarin interacts with many drugs/foods due to protein binding and CYP metabolism.
- Usability in Renal Impairment: Warfarin remains an option in severe renal dysfunction; DOACs often need caution or dose adjustment.
- Reversal: Warfarin reversed with vitamin K, PCC; DOACs have specific reversal agents but can be more expensive or less readily available.
Clinicians choose among these based on patient factors (renal function, compliance, cost, preference), indication (AF, mechanical valves), and bleeding risk.
Clinical Indications and Guidelines
Venous Thromboembolism (VTE): DVT and PE
- LMWH or fondaparinux for initial acute treatment, transitioning to warfarin or a DOAC.
- DOACs (apixaban, rivaroxaban, edoxaban, dabigatran) can be used as single-agent therapy for DVT/PE.
- Duration: Typically 3 months for provoked VTE, potentially indefinite if unprovoked or recurrent.
Atrial Fibrillation Stroke Prevention
- CHA₂DS₂-VASc score guides necessity of anticoagulation.
- DOACs often first-line in non-valvular AF, or warfarin if mechanical valve or moderate/severe mitral stenosis.
- Avoid DOACs if creatinine clearance <15 mL/min or mechanical heart valve.
Mechanical Heart Valves
- Warfarin is standard of care, with target INR depending on valve type (2.0–3.0 or 2.5–3.5).
- DOACs are generally contraindicated in mechanical heart valves after trials showed suboptimal outcomes.
Chronic Coronary Artery Disease and Peripheral Artery Disease
- Some guidelines propose low-dose rivaroxaban plus aspirin to reduce cardiovascular events in stable atherosclerotic disease.
- Balancing bleeding risk is essential.
Cancer-Associated Thrombosis
- LMWH historically considered standard.
- Recent data support certain DOACs (edoxaban, rivaroxaban) in cancer-related VTE, though LMWH or careful DOAC selection remains central.
Monitoring and Laboratory Assessment
aPTT and PT/INR
- UFH: aPTT or anti-Xa for complex cases.
- Warfarin: PT/INR for routine management.
- DOACs do not require routine lab monitoring but can influence PT/aPTT to varying extents.
Anti-Factor Xa Levels
- LMWH, rivaroxaban, apixaban, and other factor Xa inhibitors can be measured with specific anti-Xa assays if needed.
Thrombin Time / Ecarin Clotting Time
- Useful for dabigatran if necessary, though rarely done in routine care.
Clinical Monitoring
- Checking for signs of bleeding (e.g., bruising, hematuria, GI hemorrhage) is critical.
- Renal function assessed periodically to ensure correct dosing for renally cleared agents.
Adverse Effects and Bleeding Management
Generalized Risks
- All anticoagulants pose hemorrhage risk. The location and severity vary.
- Gastrointestinal bleeding is more common with factor Xa inhibitors and dabigatran.
- For major bleeds, reversing the anticoagulant effect promptly is vital.
Specific Reversal Agents and Supportive Measures
- Protamine for heparin, partial effect for LMWH.
- Vitamin K for warfarin.
- Idarucizumab for dabigatran.
- Andexanet alfa for factor Xa inhibitors.
- PCC or aPCC (activated PCC) if targeted antidotes are unavailable.
- Hemodynamic support, local measures, or surgical intervention for controlling bleeding sources.
Special Populations
- Pregnancy: LMWH is preferred. Warfarin causes teratogenicity, DOAC safety not well established.
- Elderly: Higher bleed risk, thus cautious dosing, frequent renal function checks.
- Pediatrics: LMWH can be used in children; warfarin and DOAC use is specialized.
Future Developments and Ongoing Research
Novel Mechanisms
- Factor XI inhibitors (e.g., Osocimab, Milvexian) are under investigation, potentially reducing stroke risk with diminished bleeding liability.
- Bringing forward TFPI (Tissue Factor Pathway Inhibitor) or Protein S agonists, or molecules providing more targeted attenuation of the clotting cascade.
Personalized Therapy
- Pharmacogenomic testing (e.g., CYP2C9, VKORC1 for warfarin) refines dosing.
- Monitoring biomarkers for improved risk stratification of bleeding vs. thrombosis.
- Big data and AI-driven algorithms to tailor therapy for individual patient risk profiles.
Practical Considerations and Clinical Pearls
- Choosing Drug and Route: Condition, urgency, patient compliance, renal function, and risk factors influence selection (UFH for immediate effect, LMWH for ease, DOAC for outpatient convenience, or warfarin for mechanical valves).
- Bridging Therapy: Heparin bridging with warfarin is standard for quick anticoagulation until INR reaches therapeutic range. This is not needed with DOACs due to rapid onset.
- Drug Interactions: Warfarin interacts with numerous drugs, dietary changes, and herbal supplements. DOACs often interact with strong P-gp or CYP3A4 modulators.
- Patient Education: Emphasize adherence, signs of bleeding, caution with over-the-counter NSAIDs that raise bleeding risk, and consistent follow-up.
- Cost and Access: In certain healthcare settings, warfarin remains cheaper but requires intensive monitoring. DOACs are costlier but may reduce overall healthcare burdens by limiting hospital visits for INR checks.
Conclusion
Anticoagulants stand among the most critical therapeutic tools for preventing and managing thromboembolic events, from venous clots in the legs and lungs to strokes stemming from atrial fibrillation. Traditional agents like Heparin and Warfarin have saved countless lives; however, they require diligent monitoring and caution regarding dietary or drug interactions. The advent of Direct Oral Anticoagulants (DOACs)—including Rivaroxaban, Apixaban, Edoxaban, and Dabigatran—has reshaped oral anticoagulation, making it more consistent and user-friendly, although challenges around reversal, renal impairment, and cost persist.
Key considerations in anticoagulant therapy entail selecting the appropriate agent for a given clinical indication, monitoring for potential side effects (especially bleeding), and adjusting for patient-specific factors (comorbidities, concomitant medications, genetic profiles). Ongoing research promises next-generation drugs targeting the coagulation cascade with an increasingly refined risk-benefit ratio. Through evidence-based application and vigilant patient management, clinicians can harness these agents to significantly reduce morbidity and mortality from thrombosis while minimizing hemorrhagic complications.
References
- Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 13th Edition
- Katzung BG, Basic & Clinical Pharmacology, 15th Edition
- Rang HP, Dale MM, Rang & Dale’s Pharmacology, 8th Edition
Medical Disclaimer
The medical information on this post is for general educational purposes only and is provided by Pharmacology Mentor. While we strive to keep content current and accurate, Pharmacology Mentor makes no representations or warranties, express or implied, regarding the completeness, accuracy, reliability, suitability, or availability of the post, the website, or any information, products, services, or related graphics for any purpose. This content is not a substitute for professional medical advice, diagnosis, or treatment; always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition and never disregard or delay seeking professional advice because of something you have read here. Reliance on any information provided is solely at your own risk.