I. Introduction and Historical Context
Anti-androgens are a pharmacologically diverse class of agents designed to diminish or block the effects of endogenous androgens—principally testosterone and dihydrotestosterone (DHT)—on target tissues. Since the recognition of androgen dependence in prostate cancer in the 1940s, the development of anti-androgen therapies has revolutionized management of hormone-dependent cancers, benign prostate disease, androgen excess syndromes, and more. Today’s armamentarium includes receptor antagonists, steroidogenesis inhibitors, enzyme blockers, and gonadotropin-suppressing agents, as well as emerging targeted biotech groups.
II. Physiology of Androgens
- Testosterone, the primary androgen, is synthesized in the testes (Leydig cells; 95%) and adrenal cortex (5%). Circulating testosterone is converted to the more potent DHT via 5α-reductase, especially in prostate, skin, and hair follicles.
- Actions: Androgens bind intracellular androgen receptors (AR), leading to complex gene modulation responsible for male secondary sex characteristics, muscle mass, erythropoiesis, bone density, libido, and prostate growth.
- Feedback Regulation: The hypothalamic-pituitary-gonadal axis tightly regulates androgen production via GnRH, LH, and FSH signals.
III. Rationale and Indications for Anti-Androgen Therapy
| Disease/Condition | Pathogenesis Involving Androgens | Anti-Androgen Goals |
|---|---|---|
| Prostate cancer | Growth/survival driven by AR signaling | Block AR activity, suppress androgen source |
| Benign prostatic hyperplasia (BPH) | DHT-mediated prostate hyperplasia | Reduce DHT, shrink gland |
| Hirsutism, acne, virilization (PCOS) | Androgen excess, AR hypersensitivity | Block AR, reduce androgen synthesis |
| Precocious puberty (males) | Early androgen surge | Suppress gonadal steroidogenesis |
| Transgender women (MTF) | Suppression of masculinization, feminization | Block AR, suppress endogenous testosterone |
| Paraphilias/sex offending | Hyperactive libido, inappropriate behaviors | Reduce sexual drive by anti-androgen block |
IV. Mechanisms and Major Classes of Anti-Androgens
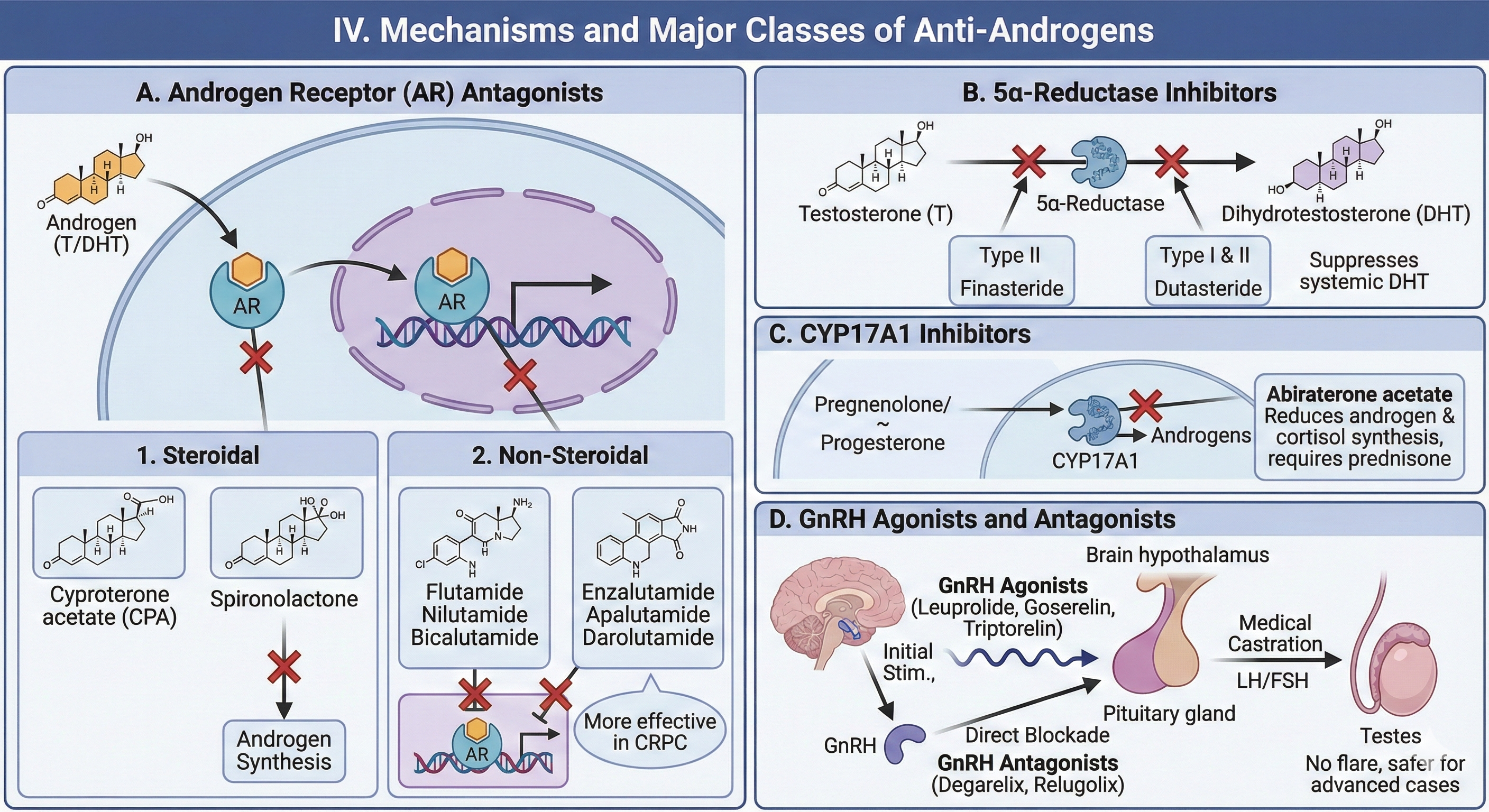
A. Androgen Receptor (AR) Antagonists
1. Steroidal Anti-Androgens
- Cyproterone acetate (CPA): Suppresses AR by competitive inhibition; has progestogenic and anti-gonadotropic properties, inhibiting pituitary LH release.
- Spironolactone: Blocks AR and inhibits androgen synthesis—used for hirsutism and acne in women; off-label for transgender therapy.
2. Non-Steroidal Anti-Androgens
- Flutamide, Nilutamide: Early, competitive AR antagonists; short half-life, hepatotoxicity risk, less used today.
- Bicalutamide: Improved selectivity, longer half-life, mild risk of hepatotoxicity—standard in prostate cancer ADT.
- Enzalutamide, Apalutamide, Darolutamide: Second-generation AR antagonists; block AR nuclear translocation and DNA binding, more effective in castration-resistant prostate cancer (CRPC). Darolutamide minimizes CNS effects due to limited blood-brain barrier penetration.
B. 5α-Reductase Inhibitors
- Finasteride: Selectively inhibits Type II isoenzyme (prostate, hair follicle); approved for BPH, male-pattern alopecia.
- Dutasteride: Dual inhibitor (Type I and II); more potently suppresses systemic DHT; used for BPH, investigated for prostate cancer prevention.
C. CYP17A1 Inhibitors
- Abiraterone acetate: Blocks CYP17A1 (17α-hydroxylase, 17,20-lyase), reducing androgen and cortisol synthesis; added with prednisone to offset mineralocorticoid excess for CRPC.
D. GnRH Agonists and Antagonists
- GnRH Agonists (Leuprolide, Goserelin, Triptorelin): Initially stimulate, then desensitize/cause receptor downregulation, suppressing LH and FSH release and testicular steroidogenesis (“medical castration”).
- GnRH Antagonists (Degarelix, Relugolix): Directly block pituitary receptors, suppressing gonadotropin and testosterone synthesis—no initial testosterone “flare,” safer for advanced cases.
V. Pharmacokinetics and Dynamics
| Agent/Class | Route | Absorption/Onset | Bioavailability | Metabolism (CYP) | Half-life | Key PK Features |
|---|---|---|---|---|---|---|
| Bicalutamide | Oral | Well absorbed | ~100% | CYP3A4 | 5–7 days | High protein binding (96%) |
| Flutamide | Oral | Rapid | ~60–70% | CYP1A2 | 5–6 h (parent) | Hepatotoxicity risk |
| Enzalutamide | Oral | High | ~85% | CYP2C8/3A4 | 6–8 days | CNS side effects possible |
| Finasteride | Oral | High | ~65% | Hepatic | ~6 h | Selective for Type II |
| Dutasteride | Oral | High | >90% | CYP3A4 | ~5 weeks | Potent, dual isoenzymes |
| Abiraterone | Oral | Must be taken fasting | ~10% | CYP3A4, SULT2A1 | ~12 h | Needs co-admin prednisone |
| Leuprolide | IM/SC depot | Controlled release | N/A | Peptide/protein | 1–6 months | Depot formulation |
| Degarelix | SC | Rapid | N/A | Peptide/protein | ~53 days | Monthly dosing |
VI. Clinical Applications
A. Prostate Cancer
- Initial (Castration-Sensitive):
- Combined androgen blockade (GnRH agonist/antagonist + AR antagonist).
- Improves survival, reduces tumor burden.
- Castration-Resistant (CRPC):
- Second-generation AR antagonists or CYP17A1 inhibitors (enzalutamide, abiraterone, apalutamide, darolutamide).
- Choice depends on comorbidities, CNS side effects, cardiovascular risk.
B. Benign Prostatic Hyperplasia (BPH)
- 5α-reductase inhibitors shrink prostate, improve urinary symptoms, slow disease progression.
C. Hirsutism, Acne, Virilization (PCOS, Ovarian/Adrenal Disorder)
- Spironolactone, flutamide, cyproterone (off-label in some regions).
- Adjunct to oral contraceptives.
D. Central Precocious Puberty
- GnRH agonist depot therapy suppresses premature sexual development.
E. Gender-Affirming Therapy
- Spironolactone, cyproterone, plus estrogens for transgender women.
- GnRH agonist/antagonist can be considered for more profound suppression.
F. Paraphilias/Sex Offending
- Cyproterone, GnRH analogues may be used under strict legal/ethical protocols.
VII. Adverse Effects and Monitoring
| Adverse Effect | Agents Most Often Implicated | Management Strategies |
|---|---|---|
| Hot flashes | GnRH analogs, AR antagonists | Lifestyle, SSRIs, gabapentin |
| Gynecomastia | Bicalutamide, flutamide, spironolactone | Tamoxifen or breast irradiation |
| Sexual dysfunction/ED | All classes (via testosterone suppression) | PDE5 inhibitors, counseling |
| Osteoporosis, fracture risk | Long-term ADT | BMD assessment, vitamin D/calcium, bisphosphonate |
| Cardiovascular risk | GnRH agonists, abiraterone | Risk assessment, modify risk factors |
| Liver toxicity | Flutamide, cyproterone, abiraterone | Regular LFTs |
| Metabolic effects | Abiraterone | Monitor potassium, blood pressure |
| CNS effects (seizure, fatigue) | Enzalutamide, apalutamide | Lower dose, switch agent |
Monitoring:
- Testosterone levels (goal <50 ng/dL for prostate cancer).
- PSA (prostate cancer surveillance).
- Bone mineral density every 1–2 years in long-term depletion.
- Liver function tests for hepatotoxic agents.
- Serum potassium, glucose for abiraterone.
VIII. Major Drug Interactions
- CYP3A4 substrates/inhibitors/inducers—potential for increased toxicity or reduced efficacy.
- Ketoconazole, rifampin alter exposure for abiraterone, bicalutamide, enzalutamide.
- QT prolonging medications: Risk additive with abiraterone, enzalutamide.
- Warfarin and anticoagulants: Potential displacement, increased bleeding risk.
IX. Emerging Therapies, Resistance, and Future Directions
- SARDs (Selective Androgen Receptor Degraders): Protein degraders that reduce AR protein, overcoming splice variant and mutation-mediated resistance.
- Novel AR antagonists: Better CNS safety, more potent at mutated AR.
- Combination strategies: ADT + chemotherapy, immunotherapy, radiopharmaceuticals (e.g., PARP inhibitors for DNA repair-deficient CRPC).
- Biotech innovations: Gene editing, siRNA approaches to androgen production or AR signaling.
X. Summary Table: Mechanism, Agent, Clinical Use, and Key Monitoring
| Mechanism | Main Agents | Clinical Uses | Key Monitoring |
|---|---|---|---|
| AR antagonism | CPA, flutamide, bicalutamide, enzalutamide, apalutamide, darolutamide | Prostate cancer, hirsutism, transgender therapy | LFTs, PSA, testosterone |
| 5α-reductase block | Finasteride, dutasteride | BPH, male-pattern hair loss | Urinary symptoms, PSA |
| Steroidogenesis block | Abiraterone | CRPC | Potassium, BP, LFTs |
| GnRH analogs | Leuprolide, goserelin, triptorelin | Prostate cancer, precocious puberty | Testosterone, BMD, anemia |
| GnRH antagonists | Degarelix, relugolix | Prostate cancer | Testosterone, ECG, BMD |
XI. Clinical Pearls and Take-home Messages
- Always monitor testosterone and PSA when treating prostate cancer with anti-androgens.
- Bone loss is a major long-term ADT risk—frequent BMD assessment and prophylaxis are essential.
- Non-steroidal AR antagonists have varying CNS penetration; select based on comorbidities.
- Abiraterone requires adjunct corticosteroid to block mineralocorticoid excess.
- All anti-androgens can cause sexual side effects—counseling and management may be required.
- QT prolongation and drug interactions often overlooked; always review concurrent medications.
- Emerging resistance mechanisms increasingly guide therapy selection and combination strategies.
XII. References
- Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 13th Edition.
- Katzung BG, Basic & Clinical Pharmacology, 15th Edition.
- Rang HP, Dale MM, Rang & Dale’s Pharmacology, 8th Edition.
- Zeind & Carvalho, Applied Therapeutics, 11th Edition.
- EAU Guidelines: Prostate Cancer, Endocrine Society Guidance.
This comprehensive, textbook-based chapter delivers mechanistic detail, clinical nuance, evidence-based recommendations, and integrated monitoring protocols—bringing authenticity and depth for advanced academic and clinical reference.
Medical Disclaimer
The medical information on this post is for general educational purposes only and is provided by Pharmacology Mentor. While we strive to keep content current and accurate, Pharmacology Mentor makes no representations or warranties, express or implied, regarding the completeness, accuracy, reliability, suitability, or availability of the post, the website, or any information, products, services, or related graphics for any purpose. This content is not a substitute for professional medical advice, diagnosis, or treatment; always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition and never disregard or delay seeking professional advice because of something you have read here. Reliance on any information provided is solely at your own risk.

