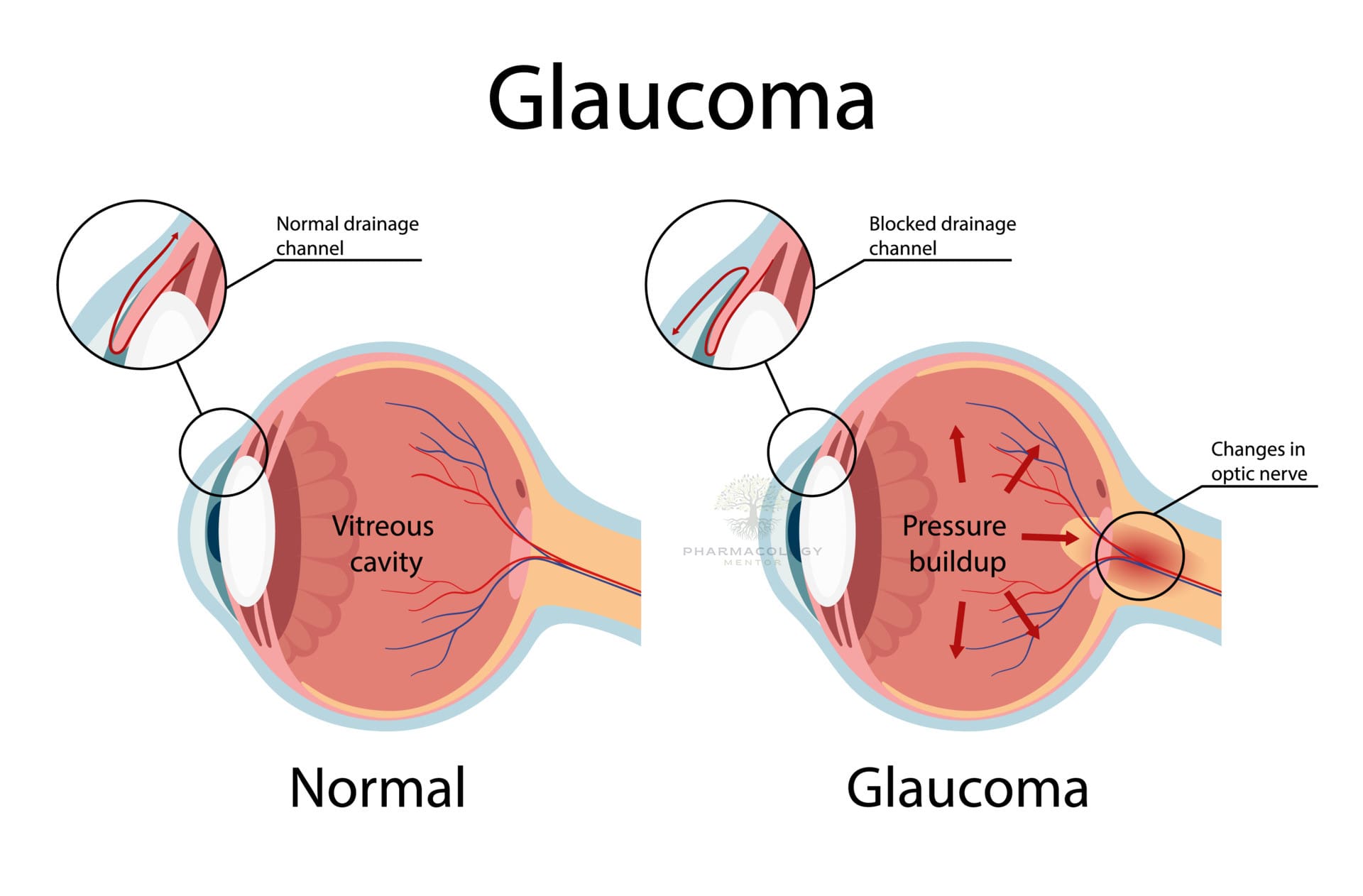
Glaucoma is a chronic, progressive optic neuropathy characterized by retinal ganglion cell loss and characteristic optic nerve/visual field damage in which lowering intraocular pressure (IOP) slows progression across open‑angle, angle‑closure, and secondary forms. The only proven disease‑modifying therapy is sustained IOP reduction via medications, laser trabeculoplasty, or surgery tailored to mechanism, severity, and risk, with acute angle‑closure managed emergently to prevent permanent vision loss.
Core definitions
Glaucoma encompasses primary and secondary disorders stratified by an open versus closed anterior chamber angle, with primary open‑angle glaucoma (POAG) the most prevalent subtype worldwide. Optic neuropathy emerges from progressive retinal ganglion cell (RGC) loss and corresponding visual field defects, often asymptomatic until advanced stages in open‑angle disease.
Epidemiology and risk
An estimated 60–70+ million people are affected globally, with numbers projected to exceed 100 million by 2040 as populations age. POAG prevalence increases with age, is higher among people of African ancestry, and is associated with risk factors including elevated IOP, family history, thin central cornea, myopia, disc hemorrhages, and low ocular perfusion pressure.
Aqueous humor physiology
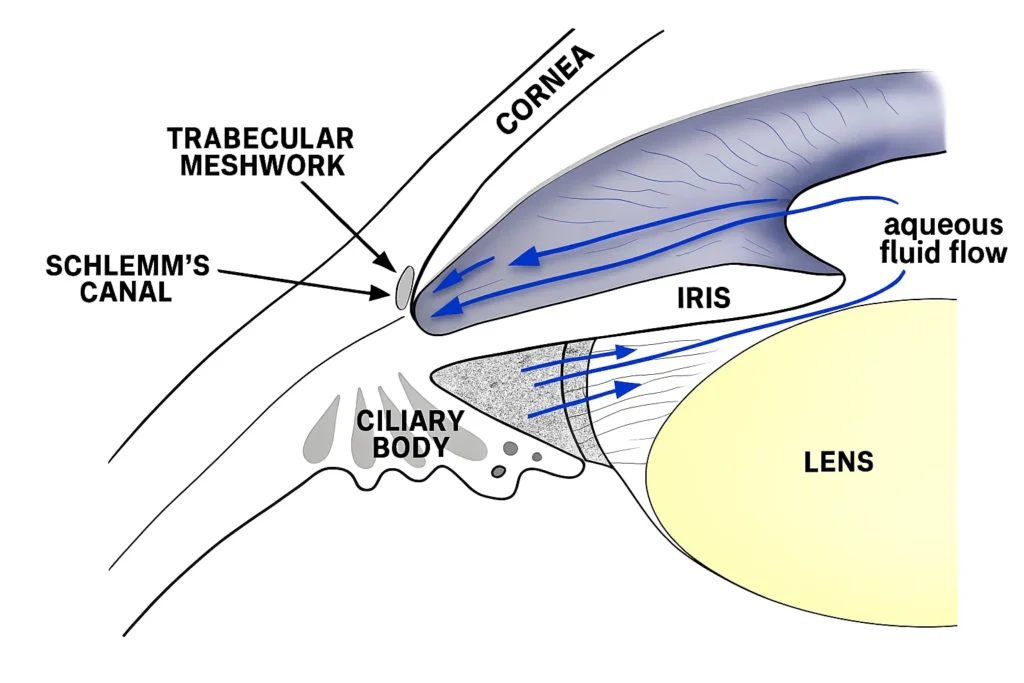
Aqueous humor is produced by the ciliary processes, flows through the pupil, and drains predominantly via the trabecular meshwork and Schlemm’s canal (conventional outflow) with a secondary uveoscleral pathway, with resistance changes determining IOP. In POAG, increased resistance at the juxtacanalicular trabecular meshwork reduces outflow and contributes to elevated IOP and diurnal fluctuation, while uveoscleral outflow declines with age.
Pathophysiology of damage
Glaucomatous optic neuropathy reflects RGC apoptosis and axonal loss with deformation of the lamina cribrosa, where mechanical strain and impaired axonal transport/vascular dysregulation contribute to progressive cupping and field loss. Although IOP is not synonymous with glaucoma, it is the key modifiable risk factor and therapeutic target across open‑angle and normal‑tension variants.
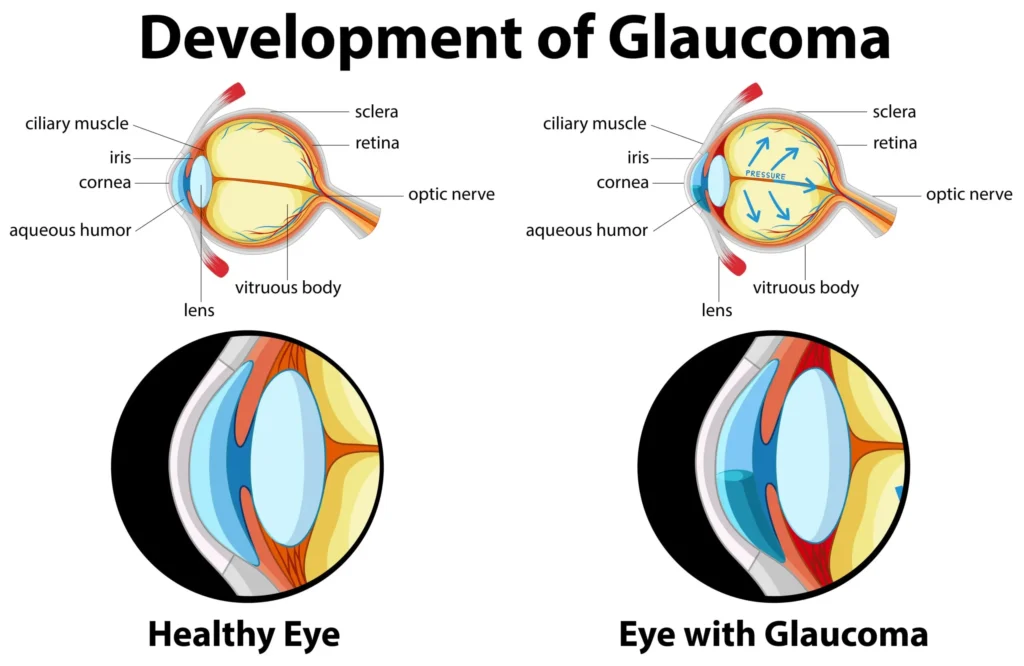
Clinical classification
- Primary open‑angle glaucoma: Open angle with increased trabecular resistance, characteristic optic nerve/RNFL loss, and corresponding field defects, often with elevated IOP but including normal‑tension presentations.
- Primary angle‑closure disease: Pupillary block and/or plateau iris mechanism narrows/closes the angle, causing acute or chronic outflow obstruction; acute angle‑closure is an ocular emergency.
- Secondary glaucomas: Pseudoexfoliative, pigmentary, neovascular, steroid‑induced, uveitic, traumatic, post‑surgical, or raised episcleral venous pressure with mechanism‑specific features and management.
Diagnosis and monitoring
Standard evaluation includes tonometry, gonioscopy, slit‑lamp and dilated fundus exam, pachymetry, perimetry, and OCT of RNFL/macula/optic nerve to confirm glaucomatous optic neuropathy and follow progression. Gonioscopy distinguishes open versus closed angles and identifies synechiae or secondary mechanisms, while target IOP is set based on baseline damage and risk with iterative adjustment over time.
Screening and ocular hypertension
The Ocular Hypertension Treatment Study demonstrated that topical hypotensive treatment delays conversion from ocular hypertension to POAG, validating IOP lowering in at‑risk eyes. USPSTF highlights uncertainty in population screening benefits but recognizes the burden of undetected disease and the importance of risk‑directed comprehensive eye examinations.
Treatment goals
Therapy aims to reach and maintain a target IOP that halts or substantially slows structural and functional progression, revisited as RNFL/visual fields evolve. IOP reduction is achieved stepwise with medications, laser trabeculoplasty, and surgery (including MIGS, filtration, and tubes), selected to maximize effectiveness, adherence, tolerability, and safety.
First‑line and adjunctive medications
- Prostaglandin analogs (e.g., latanoprost, travoprost, bimatoprost, tafluprost, latanoprostene bunod) increase uveoscleral (and NO‑enhanced conventional) outflow and are preferred initial therapy for convenience and robust 25–35% IOP reduction, with class adverse effects including conjunctival hyperemia, eyelash growth, periocular/iris pigmentation, and rare CME/uveitis.
- Beta‑blockers (e.g., timolol, levobunolol, betaxolol) reduce aqueous production by 20–25%, with systemic cautions in asthma/COPD, heart block, and bradycardia, and are useful add‑ons or alternatives when PGAs are insufficient or contraindicated.
- Alpha‑2 agonists (e.g., brimonidine) decrease production and increase uveoscleral outflow with allergy/sedation risks, especially in young children, and often serve as adjuncts.
- Topical carbonic anhydrase inhibitors (e.g., dorzolamide, brinzolamide) reduce production by ~15–20% and are additive in multi‑drug regimens, with dysgeusia/ocular surface intolerance common.
- Cholinergics (pilocarpine) increase trabecular outflow but are less tolerated (brow ache, induced myopia) and are niche in angle‑closure mechanisms or as adjuncts.
- Rho‑kinase inhibitors (netarsudil) enhance trabecular outflow and lower episcleral venous pressure, often causing conjunctival hyperemia, and can be combined with PGAs (e.g., netarsudil/latanoprost).
Laser and interventional therapies
Selective laser trabeculoplasty (SLT) lowers IOP comparably to medications and can be considered first‑line or as a drop‑sparing alternative, with randomized data (LiGHT) supporting long‑term clinical and cost‑effectiveness versus initial drops. Laser options include ALT, SLT, and micropulse techniques, with SLT favored for repeatability and lower trabecular injury.
Surgical options
- Minimally invasive glaucoma surgery (MIGS) procedures improve outflow (trabecular bypass, goniotomy, canal‑based), reduce medications, and are favored in mild‑to‑moderate disease or at the time of cataract surgery.
- Trabeculectomy and tube shunts achieve lower target IOPs for advanced disease or failure of prior therapy, with antifibrotic augmentation improving success but increasing bleb‑related complications.
- Cyclodestructive procedures (e.g., diode CPC) are reserved for refractory or poor‑vision eyes due to ciliary body ablation risks.
Acute angle‑closure glaucoma
Acute angle‑closure presents with severe eye pain, halos, corneal edema, markedly elevated IOP, mid‑dilated non‑reactive pupil, and systemic symptoms, requiring immediate IOP reduction and definitive laser peripheral iridotomy. Acute management uses rapidly acting topical agents (beta‑blocker, alpha‑2 agonist, CAI), systemic acetazolamide, hyperosmotics as needed, pilocarpine after IOP falls to break block, and urgent iridotomy in the affected and prophylactically in the fellow eye when appropriate.
Normal‑tension glaucoma
Normal‑tension glaucoma shares structural/functional features of POAG despite IOP within statistically normal ranges, with vascular dysregulation and lamina vulnerability implicated, and benefits from targeted IOP lowering and risk‑factor modification. A ~30% IOP reduction slows progression in normal‑tension eyes, supporting the target‑IOP paradigm even when baseline IOP is normal.
Follow‑up and target‑IOP strategy
Target IOP is individualized based on baseline damage, progression rate, risk factors, and life expectancy, with tighter targets for advanced disease or faster progression and iterative adjustment using OCT and visual field trends. Education on adherence, drop technique (punctal occlusion), ocular surface care, and systemic comorbidity control is central to durable control and quality of life.
High‑yield diagnostics
- Tonometry with corneal pachymetry interpretation and diurnal variability evaluation informs risk and target setting.
- Gonioscopy defines mechanism and detects synechiae/secondary causes, guiding therapy choices and laser candidacy.
- OCT of RNFL/macula and standardized perimetry provide structural–functional staging and sensitive progression detection to steer escalation.
Drug cautions and adverse effects
- Prostaglandin analogs: eyelash/iris/periorbital pigmentation, hyperemia, periorbitopathy, rare CME/uveitis reactivation; once‑daily dosing preferred.
- Beta‑blockers: systemic bronchospasm/bradycardia/AV block and fatigue; avoid nonselective agents in reactive airway or conduction disease.
- Alpha‑2 agonists: allergic blepharoconjunctivitis and CNS depression in infants/young children.
- Topical CAIs: dysgeusia, ocular irritation; systemic acetazolamide for short‑term use in spikes/acute angle‑closure.
- Rho‑kinase inhibitors: conjunctival hyperemia and corneal verticillata, with additive IOP lowering in combination therapy.
Practical algorithms
- Mild–moderate POAG: initiate PGA or SLT per patient preference/adherence profile; add beta‑blocker/alpha‑2 agonist/CAI or consider SLT/MIGS if target unmet or drops poorly tolerated.
- Advanced/rapidly progressive disease: target low‑teens IOP with combinations, SLT as appropriate, and timely filtration/tube surgery if progression persists.
- Angle‑closure spectrum: definitive LPI after acute control; manage plateau iris with adjunctive laser iridoplasty and address lens factors as indicated.
Table: Medication classes in glaucoma
Acute angle‑closure steps
- Immediate pressure lowering: topical beta‑blocker, alpha‑2 agonist, topical CAI, plus oral/IV acetazolamide; hyperosmotic agent if needed.
- Miotic (pilocarpine) after IOP falls to relieve block, followed by urgent laser peripheral iridotomy (and consider fellow‑eye prophylaxis).
- Reassess with gonioscopy to confirm angle reopening and quantify synechiae; address plateau iris or lens contribution as indicated.
Evidence anchors
Topical therapy delays OAG onset in ocular hypertension (OHTS), formalizing risk‑based initiation of drops in high‑risk suspects. Selective laser trabeculoplasty as initial therapy is clinically and cost‑effective with sustained drop‑free control in many eyes at 6 years (LiGHT), supporting earlier interventional strategies.
Patient education and systems
Patients should understand asymptomatic early disease, the necessity of lifelong monitoring, adherence to therapy, and family risk prompting screening of first‑degree relatives. Interprofessional collaboration optimizes IOP monitoring, imaging, adherence support, and timely escalation to laser or surgery to preserve vision and quality of life.
References
- Dietze J, Blair K, Zeppieri M, Havens SJ. Glaucoma. StatPearls. Treasure Island (FL): StatPearls Publishing; 2024.
- Mahabadi N, Zeppieri M, Tripathy K. Open Angle Glaucoma. StatPearls. Treasure Island (FL): StatPearls Publishing; 2024.
- Khazaeni B, Zeppieri M, Khazaeni L. Acute Angle‑Closure Glaucoma. StatPearls. Treasure Island (FL): StatPearls Publishing; 2023.
- The Ocular Hypertension Treatment Study Group. OHTS: topical therapy delays/prevents POAG onset. Arch Ophthalmol. 2002;120(6):701‑13.
- USPSTF. Screening for Primary Open‑Angle Glaucoma: Recommendation Statement. JAMA. 2022;327(20):1992–1998.
- Tripathy K. Latanoprost. StatPearls. Treasure Island (FL): StatPearls Publishing; 2024.
- Gazzard G, et al. Laser in Glaucoma and Ocular Hypertension (LiGHT) Trial: 6‑year outcomes. Ophthalmology. 2023;130(3):263–272.
Glaucoma
Medical Disclaimer
The medical information on this post is for general educational purposes only and is provided by Pharmacology Mentor. While we strive to keep content current and accurate, Pharmacology Mentor makes no representations or warranties, express or implied, regarding the completeness, accuracy, reliability, suitability, or availability of the post, the website, or any information, products, services, or related graphics for any purpose. This content is not a substitute for professional medical advice, diagnosis, or treatment; always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition and never disregard or delay seeking professional advice because of something you have read here. Reliance on any information provided is solely at your own risk.