Alzheimer’s disease is a progressive neurodegenerative disorder marked by amyloid and tau protein pathology that leads to gradual decline in memory, function, and behavior; management combines accurate staging, symptomatic cognitive therapies, comprehensive non‑pharmacologic care, and, in eligible early cases, disease‑modifying anti‑amyloid monoclonal antibodies with structured MRI safety monitoring. Evidence‑based symptomatic options include acetylcholinesterase inhibitors and memantine, while lecanemab and donanemab offer modest slowing of decline in biomarker‑confirmed early Alzheimer’s disease at the cost of amyloid‑related imaging abnormalities, requiring careful patient selection and monitoring pathways aligned with guideline resources.
Definition and burden
Alzheimer’s disease (AD) is the leading cause of dementia, characterized clinically by insidious and progressive impairment in episodic memory, language, visuospatial skills, and executive function, ultimately affecting basic activities of daily living and survival. Prevalence rises steeply with age, imposing substantial patient, caregiver, and societal burden, with recent U.S. estimates highlighting large absolute numbers across states and counties and excess mortality relative to age‑matched peers.
Pathophysiology
AD neuropathology centers on extracellular amyloid‑beta (Aβ) plaques and intracellular hyperphosphorylated tau neurofibrillary tangles, accompanied by synaptic dysfunction, neuroinflammation, and progressive network disconnection that underlie clinical decline. While amyloid is thought to initiate a cascade that enables tau pathology and neurodegeneration, clinicopathologic correlations vary, motivating biomarker‑based frameworks to define disease biology more precisely in vivo.
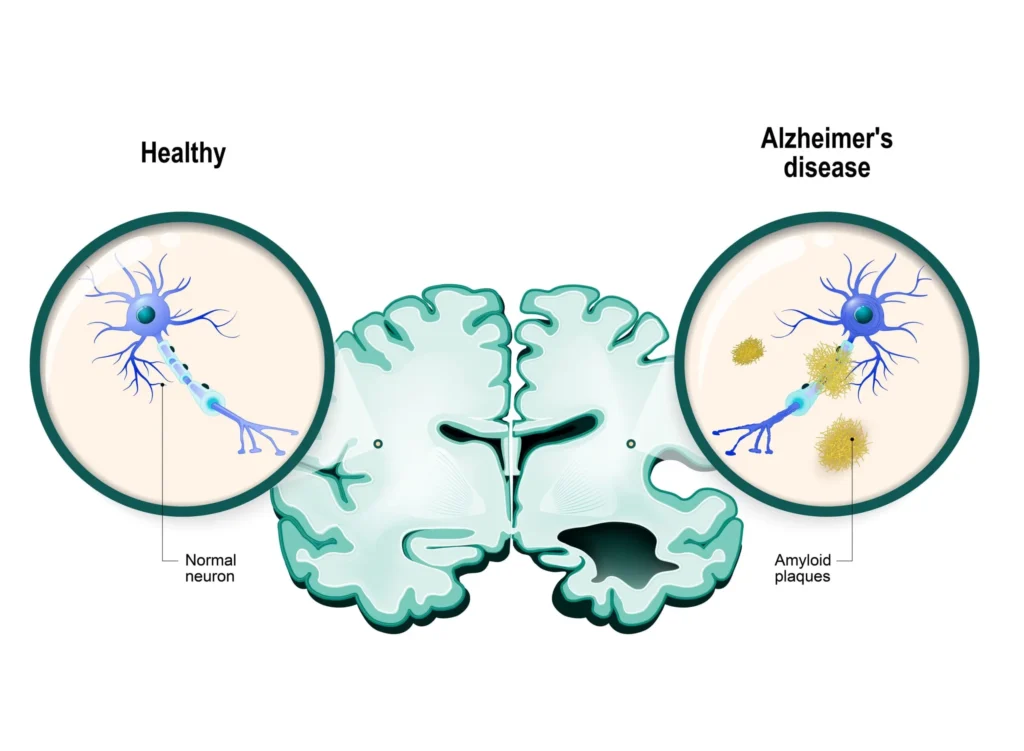
Genetics and risk
Late‑onset AD risk is strongly influenced by APOE ε4 alleles, while rare autosomal‑dominant mutations in APP, PSEN1, and PSEN2 cause early‑onset familial disease, and polygenic backgrounds further modulate lifetime probability and age of onset. Population studies underscore AD’s association with common cardiometabolic comorbidities, reinforcing the importance of vascular risk management as part of comprehensive care for cognition and overall health.
Diagnostic staging
AD spans a continuum from preclinical (biomarker‑positive, asymptomatic) to mild cognitive impairment (MCI) due to AD and mild‑to‑severe dementia due to AD, with staging supported by cognitive instruments (e.g., MMSE, MoCA), functional assessments, and caregiver input. NIA‑AA research criteria emphasize an AT(N) biomarker framework—Aβ (A), tau (T), and neurodegeneration (N)—to biologically define AD across the continuum, complementing clinical staging in research and increasingly informing therapeutic eligibility for disease‑modifying therapies.
Biomarkers and AT(N)
The AT(N) system classifies individuals using amyloid measures (CSF Aβ42 or Aβ42/40 ratio; amyloid PET), tau pathology (CSF phospho‑tau; tau PET), and neurodegeneration (MRI atrophy; FDG‑PET hypometabolism; CSF total tau) to anchor diagnosis and trial design. In symptomatic AD, A+T+ profiles are common, and biomarker confirmation of amyloid pathology is a prerequisite for anti‑amyloid monoclonal antibody therapy in early stages, aligning biological target engagement with clinical strategy.
Neuroimaging
Structural MRI supports diagnosis by excluding mimics and documenting hippocampal and temporoparietal atrophy, while FDG‑PET demonstrates posterior cingulate and parietotemporal hypometabolism consistent with AD networks. Amyloid and tau PET tracers further characterize pathology distribution, with amyloid positivity central to eligibility for anti‑amyloid therapies and to the AT(N) schema for biological classification.
Treatment goals
Therapy aims to enhance or stabilize cognition and function, manage neuropsychiatric symptoms, support safety and independence, reduce caregiver burden, and, in eligible early AD, slow clinical progression using anti‑amyloid monoclonal antibodies within safety‑governed pathways. Shared decision‑making integrates clinical stage, comorbidities, biomarker status, risk tolerance, and resource feasibility, coupled with ongoing reassessment of benefit–risk over time.
Symptomatic cognitive therapy
Acetylcholinesterase inhibitors (AChEIs)—donepezil, rivastigmine, galantamine—improve cholinergic neurotransmission and yield modest gains in cognition and global impression in mild‑to‑moderate AD, with incremental benefits in function and behavior in selected patients. Memantine, an NMDA receptor antagonist, is indicated for moderate‑to‑severe AD and can be combined with AChEIs, with pooled data supporting additive benefits on cognition, global status, activities of daily living, and neuropsychiatric symptoms in appropriate stages.

Guideline positioning and dosing
Guidance widely endorses AChEIs as options for mild‑to‑moderate AD and memantine for moderate‑to‑severe AD or AChEI intolerance, with local frameworks harmonizing initiation, titration, and shared care between specialists and primary care. Memantine is typically titrated from 5 mg daily up to 20 mg daily over several weeks with renal dose adjustments, while AChEI dosing and formulations (oral and transdermal rivastigmine) are individualized to tolerability and adherence needs.
Comparative efficacy
Individual patient data network meta‑analysis across large randomized cohorts indicates donepezil and donepezil plus memantine improve MMSE relative to placebo, with class trade‑offs in adverse events that inform dose selection and combination strategies. Decisions to continue or taper anti‑dementia drugs should consider evidence suggesting ongoing benefits—particularly with cholinesterase inhibitors—on cognition and function over longer horizons in many patients, balanced against adverse effects and goals of care.
Disease‑modifying therapies
Lecanemab and donanemab are anti‑amyloid monoclonal antibodies approved for early AD (MCI due to AD or mild dementia) that reduce amyloid burden and modestly slow clinical progression on global and cognitive composites when administered with structured MRI monitoring for ARIA safety events. U.S. approvals for lecanemab (full approval 2023) and donanemab (traditional approval July 2, 2024) reflect clinically modest but statistically significant slowing of decline in biomarker‑confirmed early disease, with programmatic pathways to manage ARIA risk and infusion logistics.
Safety and ARIA monitoring
ARIA‑E (edema/effusions) and ARIA‑H (microhemorrhages/siderosis) are key risks of anti‑amyloid therapy, heightened in APOE ε4 carriers, necessitating baseline and periodic MRI monitoring per product and professional society resources with treatment interruption or cessation thresholds for symptomatic or significant radiographic events. Clinical programs emphasize careful patient selection (confirmed amyloid positivity, early symptomatic stage), counseling regarding risks and logistics, and coordination with imaging and infusion services to ensure safe delivery and timely management of ARIA or infusion reactions.
Non‑pharmacologic management
Cognitive stimulation, structured exercise, sleep optimization, sensory optimization (vision/hearing), and caregiver education form the backbone of comprehensive care and can improve quality of life and function alongside pharmacotherapy. Safety planning, environmental support, and community resources help maintain independence and mitigate crises, while advance care planning aligns interventions with patient values as disease progresses.
Behavioral and psychological symptoms
First‑line strategies for agitation, depression, anxiety, insomnia, and psychosis prioritize non‑pharmacologic approaches (pain control, routine, engagement), with cautious, time‑limited use of psychotropics when distress or danger persists after behavioral measures. Antipsychotic use requires judicious risk–benefit appraisal due to cerebrovascular and mortality risks in dementia, and SSRIs or sleep interventions are selected with attention to anticholinergic burden and fall risk.
Vascular risk and comorbidities
Management of hypertension, diabetes, dyslipidemia, sleep apnea, and smoking is advisable for general health and may support cognitive trajectories, recognizing the frequent overlap of mixed pathologies in older adults with dementia. Comorbidity review should include polypharmacy de‑prescribing of anticholinergics and sedatives when possible, pain control, and sensory aids, each of which can meaningfully affect cognition and behavior in daily life.
Practical initiation and monitoring
Before starting AChEIs or memantine, document baseline cognition, function, neuropsychiatric status, vitals, weight, and caregiver goals, and reassess 6–12 weeks after dose stabilization for efficacy and tolerability with ongoing periodic review. For anti‑amyloid therapy, confirm amyloid positivity, evaluate MRI safety criteria, discuss ARIA risk (including APOE ε4 considerations), and schedule serial MRIs and clinical assessments aligned with professional and label‑linked resources.
When to continue or deprescribe
Continuation of AChEIs or memantine is reasonable while clinically meaningful stabilization or slowed decline persists and adverse effects are manageable, with reviews suggesting benefits to continued therapy over discontinuation across cognitive and functional outcomes, especially over the long term. Deprescribing discussions should integrate disease stage, adverse events (e.g., bradycardia, syncope, weight loss for AChEIs), adherence feasibility, and caregiver capacity, with shared decision‑making and trial discontinuations where appropriate.
Special populations
In frail older adults or those with significant cardiovascular conduction disease, AChEIs may increase syncope risk and require cautious dosing and monitoring, while the rivastigmine patch can mitigate gastrointestinal adverse effects in some patients. Moderate renal impairment necessitates memantine dose adjustments and close clinical observation, aligning with dosing frameworks in general practice guidance.
Tables
Diagnostic staging and tools
Symptomatic cognitive therapies: mechanisms, dosing, and notes
Anti‑amyloid monoclonal antibodies: candidates and safeguards
Evidence highlights
Large meta‑analyses and individual patient data syntheses substantiate that donepezil improves MMSE versus placebo and that donepezil plus memantine further improves cognitive outcomes in appropriate stages, guiding stepped pharmacotherapy in practice. Reviews of continuation versus discontinuation indicate that sustaining cholinesterase inhibitors likely benefits cognition and function over longer intervals, supporting ongoing therapy in those with perceived stabilization and tolerability.
Safety profiles
AChEIs commonly cause gastrointestinal upset, bradycardia, syncope, and weight loss, warranting slow titration and consideration of the transdermal route for rivastigmine to limit GI adverse effects in susceptible patients. Memantine is generally well tolerated, with dose adjustment for renal impairment and vigilance for dizziness or confusion during uptitration, particularly in multimorbid older adults.
Programmatic delivery of anti‑amyloid therapy
Care pathways for lecanemab and donanemab should embed patient selection (biomarker confirmation, early stage), MRI scheduling, ARIA management algorithms, infusion capacity, and education about realistic benefits and risks, supported by professional society resources. Clinics also consider APOE genotyping to inform ARIA risk discussions, though genotype does not substitute for MRI surveillance or clinical vigilance during treatment.
Non‑pharmacologic pillars
Cognitive stimulation therapies, physical activity, sleep hygiene, social engagement, and caregiver training improve quality outcomes and are integral to care plans across the AD continuum, complementing pharmacologic strategies. Environmental optimization, fall prevention, and assistive technologies support autonomy and safety, while advance care planning ensures care aligns with evolving values and capacities over time.
Practical algorithms
For mild–moderate AD, initiate an AChEI with slow titration and scheduled reassessment, adding memantine as disease progresses or earlier if mixed stages or neuropsychiatric symptoms warrant balanced by tolerability and goals. For eligible early AD with confirmed amyloid, offer anti‑amyloid monoclonal therapy within a structured program that anticipates ARIA monitoring and coordinates infusion logistics, documenting shared decisions and contingencies for adverse events.
OSCE and viva prompts
- Explain to a caregiver how donepezil may modestly improve cognition and function, what side effects to watch for, and when to reassess benefit versus risk after dose stabilization.
- Counsel a patient with MCI due to AD on eligibility steps for anti‑amyloid therapy, including amyloid confirmation, MRI scheduling, ARIA risk, and the expected magnitude of benefit in slowing progression rather than reversing disease.
Key takeaways
- Diagnosis and staging combine clinical assessment with selective biomarker use mapped to the AT(N) framework, especially when disease‑modifying therapy is contemplated in early AD.
- AChEIs and memantine provide symptomatic benefits, with combination therapy reasonable in moderate–severe disease and continuation favored when stabilization or slowed decline is perceived and adverse effects are manageable.
- Lecanemab and donanemab offer modest slowing of decline in early AD with confirmed amyloid, requiring MRI‑based ARIA surveillance, center‑level infrastructure, and informed shared decisions about risks, logistics, and goals.
📚 AI Pharma Quiz Generator
🎉 Quiz Results
Medical Disclaimer
The medical information on this post is for general educational purposes only and is provided by Pharmacology Mentor. While we strive to keep content current and accurate, Pharmacology Mentor makes no representations or warranties, express or implied, regarding the completeness, accuracy, reliability, suitability, or availability of the post, the website, or any information, products, services, or related graphics for any purpose. This content is not a substitute for professional medical advice, diagnosis, or treatment; always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition and never disregard or delay seeking professional advice because of something you have read here. Reliance on any information provided is solely at your own risk.



