Overview
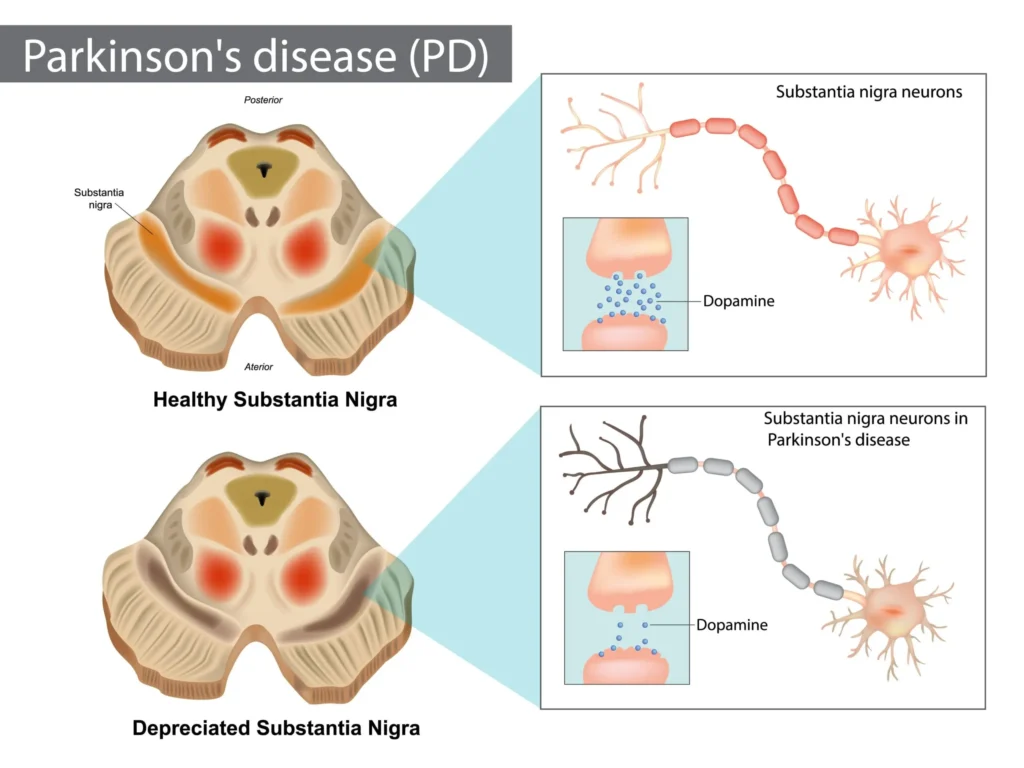
Parkinson disease (PD) is a progressive neurodegenerative disorder characterized by motor features (bradykinesia, rigidity, rest tremor, postural instability) and a wide array of nonmotor symptoms (autonomic dysfunction, sleep disorders, mood and cognitive changes, pain). The cardinal pathology is degeneration of dopaminergic neurons in the substantia nigra pars compacta with consequent striatal dopamine depletion. Pharmacotherapy aims to restore dopaminergic tone, rebalance basal ganglia circuitry, alleviate motor and nonmotor symptoms, minimize motor complications (wearing off, dyskinesias), and sustain function and quality of life. Drug classes include levodopa (with peripheral decarboxylase inhibitors), dopamine agonists, monoamine oxidase-B (MAO-B) inhibitors, catechol-O-methyltransferase (COMT) inhibitors, amantadine, centrally acting anticholinergics, and newer adjuncts such as adenosine A2A antagonists. Choice is individualized by age, symptom profile, comorbidity, and risk of adverse effects [1–4].
Pathophysiology and Pharmacologic Targets
Basal ganglia circuitry and dopamine–acetylcholine balance
- Dopamine facilitates movement through D1 receptor stimulation of the direct pathway and D2 receptor inhibition of the indirect pathway in the striatum. Loss of nigrostriatal dopamine shifts the balance toward excessive indirect pathway activity, resulting in bradykinesia and rigidity [1,2].
- Relative cholinergic overactivity contributes to tremor in some patients; this underpins the utility of anticholinergic agents for tremor-predominant PD [1–3].
- Nonmotor features reflect widespread extranigral pathology (locus coeruleus, dorsal motor nucleus of the vagus, cortex) and neurotransmitter deficits (norepinephrine, serotonin, acetylcholine), guiding symptomatic therapies (e.g., psychosis, orthostatic hypotension, sleep disturbances) [1–3].
Therapeutic goals and strategy
- Reduce motor disability and improve function with the lowest effective doses.
- Delay and mitigate motor complications by thoughtful drug selection and titration.
- Address nonmotor symptoms with targeted agents while minimizing cognitive/psychiatric adverse effects.
- Periodically reassess for dose adjustments, add-on therapies, or device-assisted approaches (e.g., intestinal levodopa gel, apomorphine infusion) as disease advances [1–4].
Classification of Antiparkinsonian Drugs
- Levodopa with peripheral decarboxylase inhibitors (carbidopa or benserazide).
- Dopamine agonists: ergot-derived (bromocriptine, cabergoline) and nonergot (pramipexole, ropinirole, rotigotine, apomorphine).
- MAO-B inhibitors: selegiline, rasagiline, safinamide.
- COMT inhibitors: entacapone, opicapone, tolcapone.
- Amantadine (IR and extended-release).
- Centrally acting anticholinergics: trihexyphenidyl, benztropine.
- Adenosine A2A receptor antagonist: istradefylline.
- Formulation-based adjuncts for “OFF” rescue: inhaled levodopa, subcutaneous apomorphine; continuous intestinal levodopa/carbidopa gel [1–4].
Levodopa and Peripheral Decarboxylase Inhibitors
Mechanism
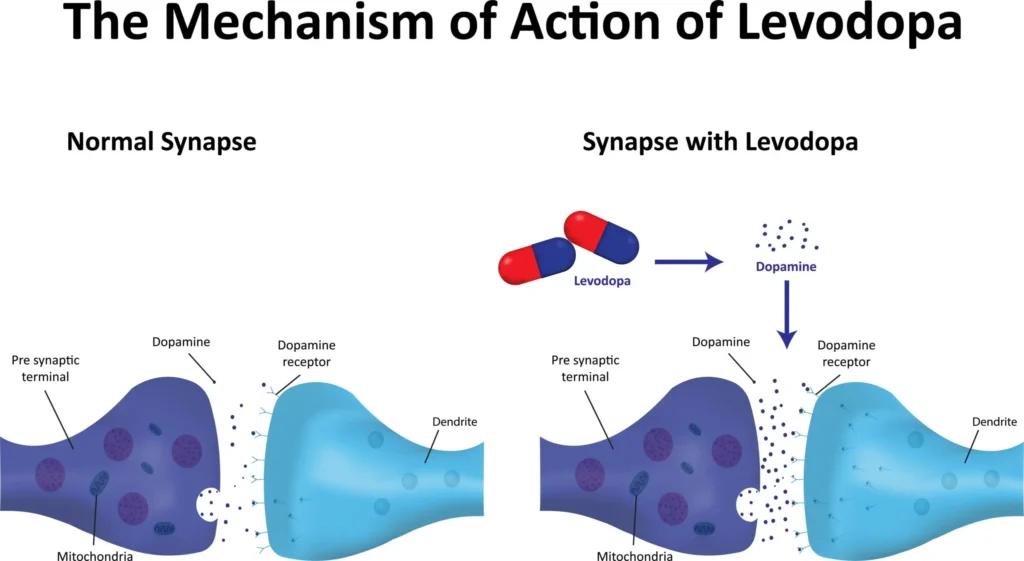
- Levodopa (L-dopa) is the metabolic precursor of dopamine. It is transported across the blood–brain barrier via the large neutral amino acid transporter and decarboxylated to dopamine by central aromatic L-amino acid decarboxylase (AADC) in surviving nigrostriatal terminals and other brain regions [1,2].
- Co-administration with a peripheral AADC inhibitor (carbidopa or benserazide) reduces peripheral metabolism, increasing CNS bioavailability and decreasing peripheral adverse effects (nausea, vomiting, orthostatic hypotension, arrhythmias) [1–3].
Formulations
- Immediate-release (IR) carbidopa/levodopa tablets (e.g., 25/100 mg).
- Controlled-release (CR) tablets (slower onset, longer duration; variable absorption).
- Extended-release (ER) capsules containing IR/ER beads (more continuous dopaminergic stimulation).
- Orally disintegrating tablets (ODT) for dysphagia.
- Inhaled levodopa powder for rapid rescue of OFF episodes.
- Levodopa–carbidopa intestinal gel (LCIG) administered via percutaneous jejunal tube for continuous infusion in advanced PD [1–4].
Pharmacokinetics
- Oral levodopa is rapidly absorbed in the small intestine; gastric emptying and competition with dietary amino acids influence absorption. Peak plasma levels occur in 0.5–2 hours (IR). Plasma half-life is ~1–2 hours with carbidopa; central effect is limited by short half-life and disease progression (shortened duration of benefit) [1,2].
- Distribution to brain depends on the transporter shared with neutral amino acids; high-protein meals can impair response (“protein effect”) [1–3].
Dosing and titration (typical)
- Start low to improve tolerability, e.g., carbidopa/levodopa 25/100 mg one-half to one tablet three times daily with meals; increase by one-half to one tablet per dose every few days to weekly based on response. Aim for daily carbidopa ≥75 mg to minimize nausea.
- Total daily levodopa dose is individualized; many patients require 300–800 mg/day early, increasing over time.
- For wearing-off, strategies include increasing dose frequency, switching to ER formulations, adding COMT or MAO-B inhibitors, or using rescue therapies [1–4].
Clinical efficacy
Levodopa remains the most effective agent for symptomatic control of bradykinesia and rigidity and improves tremor, gait, and quality of life across disease stages. Long-term use is associated with motor fluctuations and dyskinesias in many patients, particularly younger-onset PD [1–3].
Adverse effects
- Early: nausea/vomiting (area postrema stimulation), anorexia; orthostatic hypotension; cardiac arrhythmias (rare).
- Neuropsychiatric: hallucinations, vivid dreams, confusion, impulse control symptoms (less common than with dopamine agonists), somnolence.
- Motor complications: dyskinesias (peak-dose choreiform movements), wearing-off (end-of-dose akinesia), on–off phenomena, early morning OFF dystonia.
- Others: discoloration of urine/sweat; rare hyperuricemia; risk of malignant melanoma has been reported in PD cohorts (likely disease-related rather than drug-caused) [1–4].
Interactions and cautions
- Nonselective MAO inhibitors (e.g., phenelzine, tranylcypromine) combined with levodopa can precipitate hypertensive crisis—contraindicated; allow washout.
- Pyridoxine (vitamin B6) enhances peripheral decarboxylation and reduces levodopa efficacy only if levodopa is given without a decarboxylase inhibitor.
- Antipsychotics and antiemetics with D2 antagonism (e.g., haloperidol, risperidone, metoclopramide) antagonize effects and can worsen PD.
- High-protein meals can reduce response; consider protein redistribution to evening.
- Use caution in narrow-angle glaucoma, arrhythmias, peptic ulcer disease, and psychosis [1–4].
Special formulations and rescue
- Inhaled levodopa: for intermittent OFF episodes; rapid onset (~10 minutes); adverse effects include cough, upper respiratory symptoms [1–3].
- LCIG: provides continuous jejunal infusion, reducing OFF time and dyskinesia in advanced PD; procedure-related complications (tube dislodgement/infection) and device management are considerations [1–3].
Dopamine Agonists
Mechanism
Dopamine agonists directly stimulate striatal dopamine receptors (primarily D2/D3; rotigotine has broader profile), bypassing presynaptic neuronal degeneration and not requiring enzymatic conversion. They have longer half-lives than levodopa and can smooth dopaminergic stimulation [1,2].
Agents and formulations
- Nonergot: pramipexole (IR, ER), ropinirole (IR, XL), rotigotine (transdermal patch), apomorphine (subcutaneous injection and continuous infusion) [1–3].
- Ergot-derived (less used due to fibrosis/valvulopathy risk): bromocriptine, cabergoline [1–3].
Dosing (typical adult)
- Pramipexole: start 0.125 mg three times daily (IR) or 0.375 mg once daily (ER); titrate weekly to 0.5–1.5 mg TID (IR) as needed; reduce in renal impairment.
- Ropinirole: start 0.25 mg TID (IR) or 2 mg once daily (XL); titrate weekly up to 3–8 mg TID (IR) or 6–24 mg/day (XL).
- Rotigotine: start 2 mg/24 h patch; increase by 2 mg weekly to 6–8 mg/24 h (early PD) or up to 16 mg/24 h (advanced PD).
- Apomorphine: rescue injections 1–6 mg SC as needed for OFF; titrate under supervision; continuous infusion is an option in advanced PD where available [1–4].
Clinical role
- Monotherapy in early PD (especially in younger patients) to delay levodopa initiation and potentially reduce early dyskinesia risk.
- Adjunct to levodopa in advanced PD to reduce OFF time and allow levodopa dose reduction.
- Apomorphine is used as rapid rescue for sudden OFF episodes; pretreatment with an antiemetic that does not block dopamine centrally (e.g., trimethobenzamide; domperidone where available) mitigates emesis. Avoid 5-HT3 antagonists with apomorphine due to severe hypotension risk [1–4].
Adverse effects
- Nausea, vomiting (less than levodopa), orthostatic hypotension, peripheral edema.
- Neuropsychiatric: somnolence (including sudden sleep attacks), hallucinations, confusion; higher risk in elderly and with cognitive impairment.
- Impulse control disorders (ICDs): pathological gambling, hypersexuality, compulsive shopping/eating—dose-related; counsel and monitor patients and caregivers.
- Ergot-specific: pleuropulmonary and retroperitoneal fibrosis, cardiac valvulopathy; periodic echocardiography and avoidance are prudent; nonergot agents are preferred [1–4].
Interactions and cautions
- Additive hypotension with antihypertensives; sedation with CNS depressants.
- Adjust pramipexole in renal impairment; ropinirole is hepatically metabolized (CYP1A2).
- Apomorphine is contraindicated with 5-HT3 antagonists; monitor QT interval and blood pressure [1–3].
MAO-B Inhibitors
Mechanism and agents
Selective, reversible (rasagiline, safinamide) or irreversible (selegiline) inhibition of MAO-B decreases striatal dopamine breakdown, modestly improving motor symptoms and extending levodopa effect. Safinamide also modulates glutamate release, which may aid dyskinesia control [1–4].
Dosing
- Selegiline: 5 mg twice daily (morning and midday) or 1.25–2.5 mg/day as ODT/transdermal formulations in some regions; insomnia is mitigated by morning dosing.
- Rasagiline: 1 mg once daily (0.5 mg if with CYP1A2 inhibitors).
- Safinamide: start 50 mg once daily; increase to 100 mg once daily as adjunct to levodopa in fluctuating PD; avoid in severe hepatic impairment [1–4].
Clinical role
- Monotherapy in very early PD for mild symptomatic benefit.
- As adjuncts to reduce OFF time and allow levodopa dose reduction.
- Safinamide may reduce troublesome dyskinesia at 50 mg in some patients via antiglutamatergic effects [1–3].
Adverse effects and interactions
- Generally well tolerated; nausea, headache, insomnia (selegiline), dyskinesia (as dopaminergic effect increases), orthostatic hypotension.
- Risk of serotonin syndrome is low at selective MAO-B doses but caution with serotonergic agents (SSRIs/SNRIs, TCAs, tramadol, meperidine) is advised; avoid meperidine, tramadol, methadone, and dextromethorphan.
- Tyramine pressor reactions are uncommon at selective doses but advise moderation with aged/fermented foods; caution at higher doses or with loss of selectivity [1–4].
COMT Inhibitors
Mechanism and agents
COMT inhibitors reduce peripheral (entacapone, opicapone) and peripheral/central (tolcapone) metabolism of levodopa to 3-O-methyldopa, prolonging levodopa half-life and increasing its CNS availability, thereby reducing end-of-dose wearing-off [1–3].
Dosing
- Entacapone: 200 mg with each levodopa dose (up to eight times daily).
- Opicapone: 50 mg once nightly; adjust with strong CYP inducers/inhibitors as per labeling.
- Tolcapone: 100–200 mg three times daily; due to hepatotoxicity risk, reserve for refractory cases with informed consent and intensive liver function monitoring [1–4].
Adverse effects
- Dopaminergic augmentation: dyskinesia, nausea, orthostatic hypotension; manage by reducing levodopa dose.
- Diarrhea, abdominal pain; harmless orange discoloration of urine/sweat.
- Tolcapone: rare but potentially fatal hepatotoxicity; monitor LFTs frequently (e.g., every 2–4 weeks for first 6 months) and discontinue on signs of liver injury [1–3].
Amantadine
Mechanism
An antiviral agent with antiparkinsonian effects via NMDA receptor antagonism, enhanced dopamine release, and anticholinergic properties. Particularly useful for levodopa-induced dyskinesias (LID) and modestly improves bradykinesia and tremor [1–3].
Formulations and dosing
- Immediate-release (IR): 100 mg once daily, titrating to 100 mg twice to three times daily.
- Extended-release (ER) capsules/tablets: designed for once-daily dosing, with evidence for reducing LID and OFF time in advanced PD.
- Adjust dose in renal impairment; avoid abrupt discontinuation to prevent agitation or neuroleptic malignant–like syndrome [1–4].
Adverse effects
- CNS: confusion, hallucinations, insomnia, dizziness.
- Peripheral: livedo reticularis, ankle edema, dry mouth; rare skin reactions.
- Caution in elderly, cognitive impairment, and seizure disorders [1–3].
Centrally Acting Anticholinergics
Agents and role
Trihexyphenidyl and benztropine reduce cholinergic tone in the striatum, improving tremor and, to a lesser extent, rigidity. They have minimal effect on bradykinesia and are best reserved for tremor-predominant PD in younger patients without cognitive impairment [1–3].
Dosing and adverse effects
- Trihexyphenidyl: start 0.5–1 mg once or twice daily; titrate slowly to 2–6 mg/day in divided doses.
- Benztropine: 0.5–1 mg once daily up to 1–2 mg twice daily.
- Adverse effects: cognitive impairment, confusion, memory problems, blurred vision, dry mouth, constipation, urinary retention; may precipitate angle-closure glaucoma and worsen benign prostatic hyperplasia. Use extreme caution in older adults [1–4].
Adenosine A2A Receptor Antagonist
Istradefylline
- Mechanism: selective antagonism of striatal A2A receptors modulates indirect pathway activity, improving OFF time as an adjunct to levodopa without direct dopaminergic stimulation [1–3].
- Dosing: 20–40 mg once daily; reduce dose with strong CYP3A4 inhibitors; avoid with strong CYP3A4 inducers.
- Adverse effects: dyskinesia, insomnia, hallucinations, nausea; caution in psychosis [1–3].
Managing Motor Complications
Wearing-off (end-of-dose deterioration)
- Increase levodopa dosing frequency or use ER/CR formulations.
- Add COMT inhibitor (entacapone/opicapone) or MAO-B inhibitor (rasagiline/safinamide).
- Add a dopamine agonist to smooth stimulation.
- Address delayed ON by taking levodopa before meals, using ODT/IR or adding prokinetics that do not block dopamine (domperidone where available) [1–4].
On–off fluctuations and dose failures
- Consider apomorphine injections for unpredictable OFF episodes and evaluate for continuous dopaminergic therapies (LCIG, apomorphine infusion) in advanced PD.
- Optimize bowel habits and review protein intake to improve levodopa absorption [1–3].
Dyskinesias
- Peak-dose dyskinesia: reduce individual levodopa doses, increase frequency, add amantadine ER, or adjust adjuncts.
- Diphasic dyskinesia: may improve with higher levodopa dose or continuous strategies.
- OFF dystonia (e.g., early morning foot dystonia): add a bedtime CR levodopa dose or long-acting dopamine agonist; consider botulinum toxin for focal dystonia [1–3].
Freezing of gait and postural instability
- Often less levodopa responsive; physical therapy, cueing strategies, and optimization of dopaminergic therapy may help.
- Consider amantadine for gait freezing in some patients; evidence is modest [1–3].
Treatment of Nonmotor Symptoms (Selected Pharmacologic Options)
Psychosis
- First reduce or discontinue offending drugs in order: anticholinergics, amantadine, MAO-B inhibitors, dopamine agonists, then cautiously reduce levodopa if feasible.
- Pimavanserin (5-HT2A inverse agonist) treats PD psychosis without dopamine blockade; monitor for QT prolongation and interactions.
- Clozapine is effective with minimal motor worsening; requires ANC monitoring for agranulocytosis risk.
- Quetiapine is commonly used off-label, though evidence is mixed; tends to be tolerated [1–3].
Depression and anxiety
- SSRIs/SNRIs, bupropion, or mirtazapine can be used; monitor for serotonergic interactions with MAO-B inhibitors (risk is low at selective doses but counsel on symptoms).
- Cognitive-behavioral therapy and exercise are beneficial adjuncts [1–3].
Neurogenic orthostatic hypotension
- Nonpharmacologic measures: fluids/salt, compression garments, head-up tilt during sleep.
- Pharmacologic: fludrocortisone, midodrine, droxidopa (a norepinephrine precursor). Review dopaminergic regimen (agonists often exacerbate hypotension) [1–3].
Sleep disturbances
- Insomnia: sleep hygiene, melatonin; avoid sedatives that worsen cognition.
- REM sleep behavior disorder: melatonin first-line; clonazepam if refractory.
- Excessive daytime sleepiness: reduce sedating drugs; modafinil can be considered; caution with agonist-related sleep attacks [1–3].
Sialorrhea
- Glycopyrrolate or sublingual atropine drops; botulinum toxin injections into salivary glands for refractory cases [1–3].
Constipation and urinary symptoms
- Osmotic laxatives (polyethylene glycol), stool softeners, dietary fiber; avoid anticholinergic burden.
- Overactive bladder: mirabegron is preferred over antimuscarinics in cognitively vulnerable patients [1–3].
Special Populations and Clinical Considerations
Older adults and cognitive impairment
- Prefer levodopa over dopamine agonists and anticholinergics due to lower risk of hallucinations, edema, and ICDs.
- Avoid anticholinergics; use amantadine cautiously. Start low, go slow; monitor for confusion and psychosis [1–3].
Younger-onset PD
- Consider initial dopamine agonist or MAO-B inhibitor to delay levodopa-related motor complications; balance against higher ICD risk with agonists.
- Early incorporation of exercise and education on ICDs is essential [1–3].
Comorbidities
- Psychiatric disease: avoid or minimize agonists and anticholinergics; prefer levodopa.
- Cardiovascular/autonomic failure: monitor orthostatic blood pressure; adjust regimens; consider droxidopa/midodrine.
- Renal impairment: adjust pramipexole and amantadine; entacapone/opicapone are primarily hepatic; tolcapone requires hepatic vigilance [1–4].
Perioperative and anesthesia
- Maintain dopaminergic therapy perioperatively (via ODT, NG tube, or transdermal rotigotine) to prevent severe rigidity or neuroleptic malignant–like syndromes.
- Avoid dopamine-blocking antiemetics (metoclopramide, prochlorperazine); use ondansetron cautiously (avoid with apomorphine).
- Avoid meperidine or tramadol with MAO-B inhibitors [1–3].
Pregnancy and lactation
- Data are limited; levodopa/carbidopa has the most experience and may be considered if benefits outweigh risks.
- Avoid ergot agonists (risk of fibrosis/valvulopathy) and anticholinergics when possible; weigh risks of untreated PD vs drug exposure. Shared decision-making is essential [1–3].
Drug Interaction Summary
- Levodopa: antagonized by D2 blockers; contraindicated with nonselective MAO inhibitors; protein competes with absorption; iron may chelate and reduce absorption—separate administration.
- Dopamine agonists: additive hypotension with antihypertensives; CNS depression with sedatives; apomorphine contraindicated with 5-HT3 antagonists.
- MAO-B inhibitors: caution with serotonergic drugs (risk of serotonin syndrome), dextromethorphan, and certain opioids (meperidine, tramadol, methadone); modest tyramine sensitivity at therapeutic doses.
- COMT inhibitors: increase levodopa exposure—anticipate need to reduce levodopa dose; tolcapone hepatotoxic interactions with other hepatotoxins.
- Amantadine: additive anticholinergic/CNS effects; dose adjust in renal impairment.
- Anticholinergics: additive antimuscarinic burden; contraindicated in narrow-angle glaucoma and urinary retention.
- Istradefylline: CYP3A4 substrate—dose adjust with inhibitors/inducers [1–4].
Comparative Efficacy and Choosing Initial Therapy
Symptom control
- Levodopa provides the greatest motor benefit and remains the gold standard for bradykinesia/rigidity.
- Dopamine agonists and MAO-B inhibitors give modest benefit as monotherapy in early PD and are valuable adjuncts to levodopa in advanced PD.
- Amantadine is the agent of choice for levodopa-induced dyskinesia; anticholinergics are reserved for tremor-predominant PD in younger patients [1–3].
Personalizing therapy
- Age <60–65 years with mild symptoms: consider MAO-B inhibitor or dopamine agonist monotherapy; add levodopa when disability warrants.
- Age ≥60–65 years or significant functional impairment: initiate levodopa; add adjuncts if wearing-off develops.
- Tremor-predominant: consider anticholinergic (young) or add-on propranolol in selected cases; optimize levodopa/dopamine agonist.
- Prominent fluctuations: use ER levodopa, add COMT/MAO-B inhibitor or agonist; consider amantadine for dyskinesia; escalate to LCIG/apomorphine infusion if refractory [1–4].
Practical Prescribing and Monitoring
Titration and follow-up
- Start with low doses and titrate weekly based on efficacy and tolerability.
- Review wearing-off patterns, dyskinesias, sleepiness, hallucinations at each visit; use patient diaries for OFF time.
- Monitor blood pressure (orthostasis), impulse control behaviors (at baseline and periodically), and liver enzymes with tolcapone [1–3].
Patient education
- Dose timing relative to meals (levodopa 30–60 minutes before food when possible; manage protein distribution).
- Recognize and report ICDs, hallucinations, sudden sleep attacks.
- Avoid abrupt cessation of dopaminergic therapies (risk of severe rigidity, NMS-like syndrome) [1–3].
Adherence strategies
- Simplify regimens with ER formulations or transdermal systems; consider combination products (e.g., levodopa/carbidopa/entacapone).
- For rescue needs, train patients/caregivers in inhaled levodopa or SC apomorphine administration [1–3].
Selected Drug Profiles
Carbidopa/Levodopa
- Strengths: most effective symptomatic therapy; broad motor benefit; multiple formulations including ER and intestinal gel.
- Limitations: motor complications over time; nausea, orthostasis, hallucinations; dietary interactions; short half-life necessitating frequent dosing (for IR) [1–4].
Pramipexole
- Strengths: effective as mono- or adjunct therapy; renal clearance simplifies interactions.
- Limitations: somnolence/sleep attacks, edema, ICDs; dose adjust in renal impairment; hallucinations in elderly [1–3].
Ropinirole
- Strengths: flexible dosing, XL once daily option; effective adjunct to reduce OFF.
- Limitations: CYP1A2 interactions (e.g., ciprofloxacin); similar adverse profile to pramipexole [1–3].
Rotigotine
- Strengths: continuous transdermal delivery; useful for swallowing difficulties and nocturnal symptoms.
- Limitations: application-site reactions; class-related adverse effects (ICDs, somnolence) [1–3].
Apomorphine
- Strengths: rapid, reliable rescue for OFF episodes; infusion option for advanced PD.
- Limitations: emesis, hypotension, QT prolongation; requires antiemetic prophylaxis; contraindication with 5-HT3 antagonists [1–3].
Selegiline
- Strengths: inexpensive; mild symptomatic benefit; adjunct to reduce OFF.
- Limitations: insomnia, amphetamine-like metabolites; serotonergic cautions [1–3].
Rasagiline
- Strengths: once daily; fewer activating metabolites than selegiline; adjunct efficacy for OFF time.
- Limitations: serotonergic cautions; dyskinesia/orthostasis as dopaminergic effects increase [1–3].
Safinamide
- Strengths: adjunctive reduction of OFF and possible antidyskinetic effect at lower doses; once daily.
- Limitations: hepatic impairment contraindication; serotonergic cautions; visual disturbances reported [1–3].
Entacapone
- Strengths: reduces wearing-off with each levodopa dose; available in combination tablets with levodopa/carbidopa.
- Limitations: diarrhea, urine discoloration; increases dyskinesia (requires levodopa dose reduction) [1–3].
Opicapone
- Strengths: once-nightly dosing; effective OFF-time reduction; well tolerated.
- Limitations: similar class adverse effects; interactions via CYP pathways possible [1–3].
Tolcapone
- Strengths: potent COMT inhibition including central; robust effect on OFF time.
- Limitations: hepatotoxicity risk necessitating stringent monitoring; diarrhea; not first-line [1–3].
Amantadine (IR/ER)
- Strengths: best available pharmacologic therapy for levodopa-induced dyskinesia; reduces OFF time in ER forms.
- Limitations: hallucinations, edema, livedo reticularis; renal dose adjustments [1–3].
Trihexyphenidyl/Benztropine
- Strengths: useful for tremor in younger patients.
- Limitations: cognitive and autonomic adverse effects limit use, especially in older adults [1–3].
Istradefylline
- Strengths: adjunct to reduce OFF without direct dopaminergic stimulation.
- Limitations: insomnia, hallucinations, dyskinesia; CYP3A4 interactions [1–3].
Suggested Adult Dosing Summary (Typical)
- Carbidopa/levodopa IR: start 25/100 mg 0.5–1 tablet TID; titrate. ER capsules allow 2–3 times daily dosing; inhaled levodopa 84 mg per dose as needed for OFF.
- Pramipexole: IR 0.125 mg TID titrated to effect (up to ~1.5 mg TID); ER 0.375 mg daily titrated weekly.
- Ropinirole: IR 0.25 mg TID up to 3–8 mg TID; XL 2 mg daily up to 24 mg daily.
- Rotigotine: 2 mg/24 h patch, increase by 2 mg weekly to 6–8 mg (early) or up to 16 mg (advanced).
- Apomorphine: SC 1–6 mg per OFF episode; titrate; consider continuous infusion in advanced PD where available.
- Selegiline: 5 mg morning and midday; avoid late dosing.
- Rasagiline: 1 mg once daily.
- Safinamide: 50 mg once daily, may increase to 100 mg once daily.
- Entacapone: 200 mg with each levodopa dose.
- Opicapone: 50 mg once nightly.
- Tolcapone: 100–200 mg TID with LFT monitoring.
- Amantadine IR: 100 mg daily to 100 mg BID–TID; ER: once daily per product labeling.
- Trihexyphenidyl: 0.5–1 mg daily, titrate to 2–6 mg/day in divided doses.
- Istradefylline: 20–40 mg once daily [1–4].
Key Takeaways
- Levodopa remains the most effective symptomatic therapy for PD; adjuncts are layered to manage fluctuations and dyskinesias as disease progresses.
- Dopamine agonists can delay levodopa use in younger patients and reduce OFF time but carry risks of somnolence and impulse control disorders.
- MAO-B and COMT inhibitors extend levodopa benefit and reduce wearing-off; tolcapone requires stringent hepatic monitoring.
- Amantadine is the pharmacologic mainstay for levodopa-induced dyskinesia.
- Anticholinergics are reserved for tremor-predominant PD in younger patients; avoid in older adults.
- Newer adjuncts (istradefylline, inhaled levodopa, apomorphine rescue/infusion, LCIG) expand options for OFF management in advanced PD.
- Individualize therapy, start low and titrate, educate patients on adverse effects (ICDs, hallucinations, sleep attacks), and reassess regularly to optimize outcomes [1–4].
References
- Brunton LL, Hilal-Dandan R, Knollmann BC, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 14th ed. New York: McGraw-Hill Education; 2022.
- Katzung BG, Kruidering-Hall M, Trevor AJ. Basic & Clinical Pharmacology. 15th ed. New York: McGraw-Hill Education; 2021.
- Ritter JM, Flower RJ, Henderson G, Loke YK, MacEwan DJ, Rang HP, et al. Rang & Dale’s Pharmacology. 9th ed. London: Elsevier; 2019.
- Tripathi KD. Essentials of Medical Pharmacology. 8th ed. New Delhi: Jaypee Brothers Medical Publishers; 2018.
Medical Disclaimer
The medical information on this post is for general educational purposes only and is provided by Pharmacology Mentor. While we strive to keep content current and accurate, Pharmacology Mentor makes no representations or warranties, express or implied, regarding the completeness, accuracy, reliability, suitability, or availability of the post, the website, or any information, products, services, or related graphics for any purpose. This content is not a substitute for professional medical advice, diagnosis, or treatment; always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition and never disregard or delay seeking professional advice because of something you have read here. Reliance on any information provided is solely at your own risk.

