Introduction
Discovered almost a century ago, insulin remains a cornerstone of therapy for diabetes mellitus—a global health challenge characterized by hyperglycemia, insulin resistance, and/or insufficient insulin secretion. Beyond diabetes, insulin also plays pivotal roles in metabolic research and critical care (e.g., hyperkalemia management). Fundamentally, insulin drives glucose uptake into muscle and adipose tissue, regulates hepatic glucose output, and influences fat and protein metabolism.
This comprehensive analysis explores the pharmacology of insulin, referencing principal pharmacology texts like Goodman & Gilman’s The Pharmacological Basis of Therapeutics (13th Edition), Katzung BG, Basic & Clinical Pharmacology (15th Edition), and Rang & Dale’s Pharmacology (8th Edition). Discussions will include insulin’s history, synthesis, structure, mechanism of action, pharmacokinetics, classifications (rapid-, short-, intermediate-, and long-acting), therapeutic uses, complications, special populations, and emerging developments in insulin therapy.
Historical Overview
Insulin’s discovery in 1921 by Dr. Frederick Banting, Charles Best, and colleagues revolutionized the management of Type 1 Diabetes Mellitus. Prior to this, diabetes was nearly a fatal illness. The isolation of insulin from the pancreatic islets and its successful use in humans delivered a landmark shift in endocrinology. Early formulations relied on bovine or porcine pancreas extracts, but with the advent of recombinant DNA technology in the 20th century came human insulin analogs, improved purity, and reduced immunogenicity (Goodman & Gilman’s). Progress continued with the creation of engineered insulin analogs to closely approximate physiologic insulin profiles, culminating in an ever-growing array of preparations.
Endogenous Insulin: Physiology and Synthesis
Production Process in the Pancreas
In a normal pancreas, beta cells of the islets of Langerhans synthesize proinsulin—a single-chain precursor. Proinsulin is composed of the alpha (A) and beta (B) chains linked by a connecting peptide (C-peptide). Proteolytic cleavage within the Golgi yields mature insulin (the A and B chains joined by disulfide bonds) plus free C-peptide. Both are stored in secretory granules and secreted in roughly equimolar quantities upon stimulation (Katzung).
Regulation of Secretion
Glucose is the principal regulator of insulin release. Following a meal, elevated blood glucose is transported into beta cells via GLUT2 transporters (in humans, the isoform is akin but some differences exist). This leads to increased intracellular ATP, inhibition of ATP-sensitive potassium channels, depolarization, calcium influx, and exocytosis of insulin-containing granules (Rang & Dale’s). Other nutrients (amino acids, fatty acids) and incretin hormones (e.g., GLP-1, GIP) also modulate insulin secretion. Conversely, epinephrine and sympathetic tone can dampen release.
Insulin release
Insulin release is a complex physiological process that involves the coordination of various cells and signaling pathways within the pancreas. It primarily occurs in response to changes in blood glucose levels. The primary role of insulin is to regulate glucose metabolism in the body by facilitating the uptake of glucose into cells, particularly muscle and adipose (fat) cells. Here’s an overview of the mechanism of insulin release:
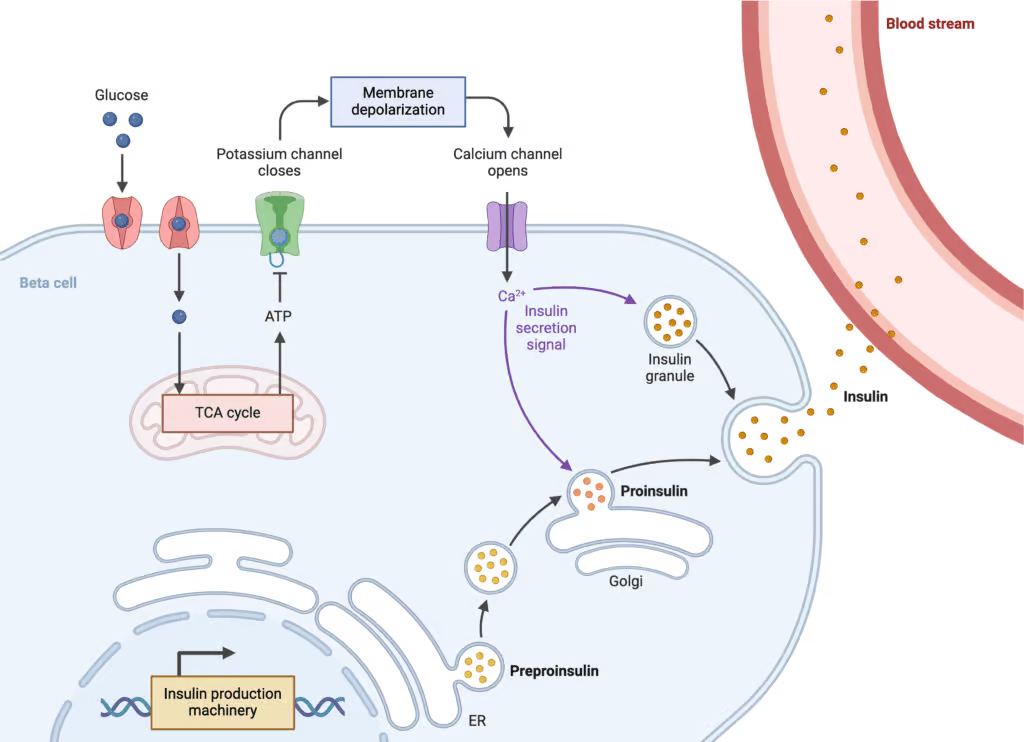
- Glucose Entry into Beta Cells: Glucose enters the beta cells of the pancreatic islets (also known as the islets of Langerhans) through glucose transporters, primarily GLUT2. Beta cells are specialized cells within the pancreas responsible for producing and releasing insulin.
- Glucose Metabolism: Inside the beta cells, glucose undergoes glycolysis, a process that breaks down glucose to produce energy in the form of adenosine triphosphate (ATP). This metabolic process generates an increase in the intracellular ATP-to-adenosine diphosphate (ADP) ratio.
- Closure of ATP-Sensitive Potassium Channels: The increased ATP-to-ADP ratio in the beta cells leads to the closure of ATP-sensitive potassium (KATP) channels in the cell membrane. These channels are normally open and help maintain the resting membrane potential of the cell.
- Membrane Depolarization: Closure of KATP channels results in membrane depolarization due to a decrease in potassium efflux. This depolarization opens voltage-gated calcium channels (VGCCs) in the cell membrane.
- Calcium Influx: The opening of VGCCs allows calcium ions (Ca2+) to enter the beta cells from the extracellular space. The influx of calcium triggers exocytosis of insulin-containing vesicles, also known as insulin granules.
- Insulin Secretion: The insulin granules fuse with the cell membrane and release insulin into the bloodstream through a process called exocytosis. Insulin is then carried by the bloodstream to target tissues, such as muscle and adipose tissue.
It’s important to note that insulin release is not solely regulated by glucose levels. Other factors, such as hormonal signals and neurotransmitters, also play a role. For instance, incretin hormones (such as glucagon-like peptide-1 or GLP-1) are released from the intestines in response to food intake. They enhance insulin release in a glucose-dependent manner by stimulating beta cells.
Overall, the mechanism of insulin release involves intricate interplay between glucose metabolism, ion channels, calcium signaling, and vesicle exocytosis. This process ensures that insulin is released when blood glucose levels rise, allowing for the proper regulation of glucose metabolism throughout the body.
Physiological Actions
- Glucose Uptake Enhancement: Insulin stimulates GLUT4 translocation in muscle/adipose tissues, allowing postprandial glucose influx.
- Glycogen Synthesis: By activating glycogen synthase, insulin fosters hepatic and skeletal muscle glycogen storage.
- Lipogenesis: Insulin upregulates lipoprotein lipase, facilitating free fatty acid entry into adipocytes.
- Protein Anabolism: Acceleration of amino acid uptake and protein synthesis in muscle.
- Inhibition of Gluconeogenesis and Lipolysis: Minimizing hepatic glucose output and lipolysis under insulin’s influence.
In diabetes, insulin deficiency or resistance compromises these processes, leading to hyperglycemia and metabolic derangements.
Chemistry and Structure of Insulin
Mature insulin comprises two polypeptide chains—an A chain (21 amino acids) and a B chain (30 amino acids)—linked by two disulfide bridges, plus an intrachain disulfide in the A chain. In solution, insulin can form dimers or hexamers (particularly in the presence of zinc). These oligomeric states profoundly influence insulin’s pharmacokinetics—foundation for different formulations’ onset and duration (Goodman & Gilman’s).
Mechanism of Action
Insulin Receptor and Signal Transduction
Insulin exerts effects by engaging a transmembrane receptor tyrosine kinase (RTK). Binding of insulin to the receptor’s alpha subunits triggers autophosphorylation in beta subunits, activating downstream signaling cascades:
- IRS (Insulin Receptor Substrate) Proteins: Phosphorylated IRS proteins recruit phosphatidylinositol 3-kinase (PI3K), prompting PIP2 → PIP3 conversion.
- Protein Kinase B (Akt) Pathway: Activated Akt orchestrates glycogen synthesis, GLUT4 translocation, and protein synthesis.
- Ras/MAPK Pathway: Influences cell proliferation and growth (Katzung).
GLUT4 Translocation
A hallmark effect in muscle and adipose tissue is the movement of GLUT4 glucose transporters from cytoplasmic vesicles to the plasma membrane, raising glucose uptake up to 20-fold.
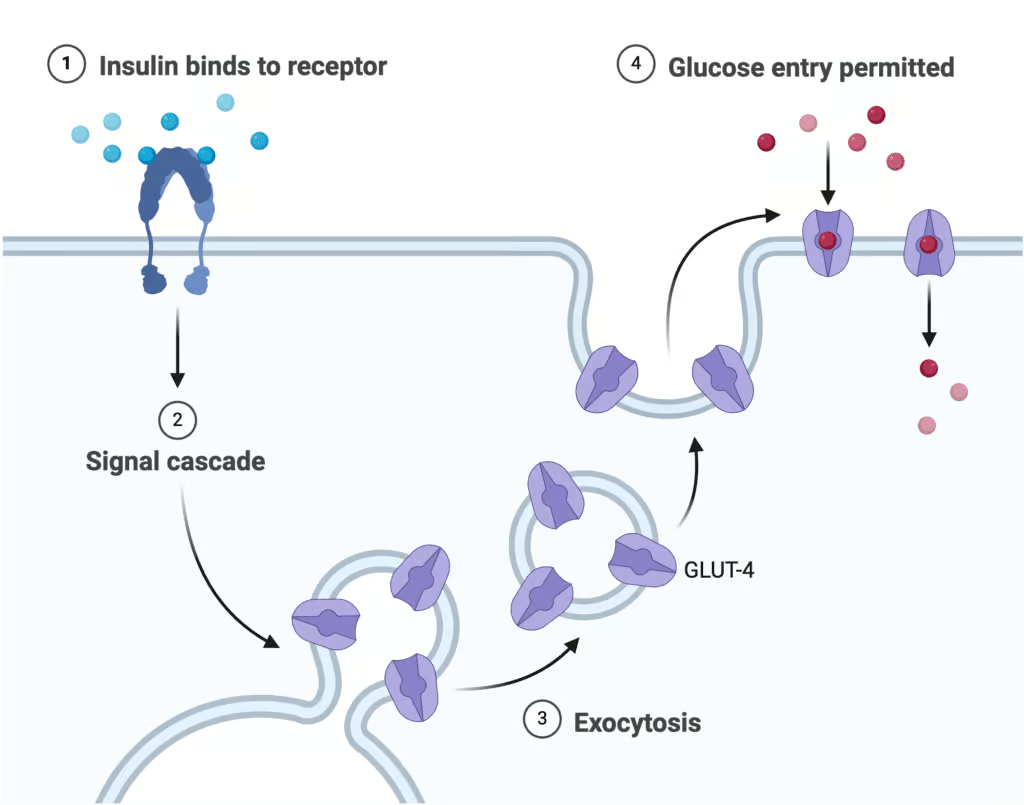
Explanation
- Insulin binds to the Insulin Receptor (IR) on the cell membrane.
- This activates PI3 Kinase (PI3K), triggering the formation of PIP3.
- PIP3 activates AKT Kinase (AKT), which stimulates GLUT4 Translocation.
- GLUT4 facilitates Glucose Uptake into the cell.
Pharmacokinetics of Exogenous Insulin
Absorption
Exogenous insulin must be injected subcutaneously (or less commonly, IV/IM). Absorption rates hinge on factors including site of injection (abdomen > arm > thigh > buttock), local blood flow, formulation characteristics (e.g., hexameric vs. monomeric states), and patient technique (Rang & Dale’s).
Distribution
Insulin circulates unbound; the majority is metabolized in liver, kidney, and muscle by insulin-degrading enzyme. Renal impairment may prolong insulin’s half-life, necessitating dose adjustments.
Routes of Administration
- Subcutaneous: Most common, with varying onset/duration across different preparations.
- Intravenous: For critical hyperglycemia management (diabetic ketoacidosis, hyperosmolar states). Regular insulin or rapid analogs may be used in infusion.
- Inhalational: An alternative route for mealtime coverage (e.g., Afrezza), though acceptance and consistent absorption remain under study.
Classification and Types of Exogenous Insulin
Formulations differ in structural modifications, leading to varied onset, peak, and duration. According to the standard nomenclature:
1. Rapid-Acting Insulins
Examples: Insulin Lispro, Insulin Aspart, Insulin Glulisine.
These analogs have minimal tendency to self-associate into hexamers. They begin action within 15 minutes, peak at ~1 hour, and last 2–4 hours (Katzung). Ideal for prandial coverage, these can be injected just before meals.
2. Short-Acting (Regular) Insulin
Example: Regular Human Insulin (Humulin R, Novolin R).
When injected subcutaneously, onset ~30 minutes, peak 2–3 hours, duration up to 6 hours. The hexameric form delays absorption relative to rapid analogs. Intravenous infusion of regular insulin is standard for diabetic ketoacidosis or perioperative glycemic control (Goodman & Gilman’s).
3. Intermediate-Acting Insulins
Example: Neutral Protamine Hagedorn (NPH).
Combining insulin with protamine and zinc yields a cloudy suspension with slower absorption. Onset ~2 hours, peak 6–10 hours, total action up to 16 hours. Often used twice a day in type 2 diabetes regimens with or without short-acting coverage.
4. Long-Acting (Basal) Insulins
Examples: Insulin Glargine, Insulin Detemir, Insulin Degludec.
- Glargine: Precipitates in subcutaneous tissue, releasing steadily over ~24 hours without a pronounced peak.
- Detemir: Binds albumin, prolonging half-life, typically dosed once or twice daily.
- Degludec: Forms multi-hexamers subcutaneously, extremely long half-life (>24 hours), enabling flexible daily injection times with minimal hypoglycemia risk (Rang & Dale’s).
5. Pre-Mixed Formulations
Combine intermediate with rapid or short-acting in fixed ratios (e.g., 70% NPH/30% regular). Convenient but less flexible for dosing adjustments.
Therapeutic Uses and Regimens
Type 1 Diabetes Mellitus
Absolute insulin deficiency from autoimmune beta-cell destruction necessitates exogenous insulin for survival. Intensive insulin therapy attempts to replicate normal physiology via basal insulin plus mealtime (bolus) coverage. Continuous Subcutaneous Insulin Infusion (CSII) with a pump is an option for enhanced glycemic precision (Goodman & Gilman’s).
Type 2 Diabetes Mellitus
Upon progression of beta-cell failure, or inadequate control on oral agents, insulin might be added. Basal insulin (e.g., Glargine or Detemir) is often initiated first, sometimes combined with oral antidiabetics. Eventually, pre-meal boluses or intensive regimens can be introduced for comprehensive control (Katzung).
Diabetic Ketoacidosis (DKA) and Hyperosmolar States
IV regular insulin infusion corrects hyperglycemia and halts ketogenesis. Careful electrolyte monitoring (particularly potassium) is essential.
Gestational Diabetes
Insulin is the gold standard if diet/exercise fail to maintain euglycemia. Rapid and intermediate formulations are typically used carefully to avoid fetal hypoglycemia.
Hyperkalemia
Insulin (with glucose) drives potassium into cells, temporarily reducing serum K⁺. This is crucial for acute hyperkalemia management. Co-administration of dextrose prevents hypoglycemia (Rang & Dale’s).
Dosage Strategies and Administration
Multiple Daily Injections (MDI)
Basal insulin once or twice daily plus rapid-acting prior to meals. This approach tries to mimic endogenous fluctuations. Carbohydrate counting and insulin-to-carb ratios facilitate flexible meal planning.
Continuous Subcutaneous Insulin Infusion (Insulin Pump)
An insulin pump delivers rapid-acting analog at a basal rate, with patient-activated boluses. Provides finer glycemic control, beneficial for motivated patients or those with frequent hypoglycemia unawareness.
Sliding Scale vs. Correction Factor
Older “sliding scale” approaches use only reactive dosing. In modern practice, correction factor insulin is added to scheduled basal-bolus doses, more effectively addressing hyperglycemia while minimizing swings.
Injection Technique
Rotation of injection sites (abdomen, thigh, buttock, arm) prevents lipodystrophy or malabsorption. Proper needle length, angle, and ensuring subcutaneous placement are crucial for consistent absorption (Katzung).
Complications and Adverse Effects
Hypoglycemia
The most significant adverse event, often from excessive dosing, missed meals, or increased physical activity. Symptoms range from adrenergic (tachycardia, tremors, sweating) to neuroglycopenic (confusion, seizures, coma). Patients should have quick access to glucose or glucagon kits, with self-monitoring of blood glucose (SMBG) to prevent episodes (Goodman & Gilman’s).
Weight Gain
Improved glycemic control under exogenous insulin can lead to anabolic effects, promoting adipose deposition especially if caloric intake is not balanced.
Lipodystrophy
Chronic injection into the same site can cause lipohypertrophy (localized fat accumulation) or lipoatrophy (fat loss). Rotating sites and using purified human insulins decrease these issues.
Allergic Reactions
Human insulin analogs drastically reduced immunogenicity. True insulin allergy is rare, though local injection-site reactions or pruritus can occur.
Insulin Edema
Occasionally, abrupt normalization of hyperglycemia triggers fluid retention and peripheral edema, more common if severe hyperglycemia was chronic.
Drug Interactions
- Beta-blockers can mask hypoglycemia’s adrenergic warnings.
- Corticosteroids, sympathomimetics, or thyroid hormones may necessitate increased insulin dose.
- Alcohol can potentiate hypoglycemia or hamper gluconeogenesis (Rang & Dale’s).
Special Populations
Pediatric Patients
Young children often need smaller doses, frequent SMBG, and vigilant adult supervision. Insulin pumps can help manage erratic eating patterns. Minimizing hypoglycemia is paramount for normal growth and cognitive development.
Elderly Patients
Comorbidities, polypharmacy, and reduced hypoglycemia awareness complicate insulin therapy. Simplifying regimens, focusing on safety over strict targets, and avoiding severe hypoglycemia are central geriatric aims (Katzung).
Renal Impairment
As the kidney contributes to insulin clearance, advanced renal dysfunction prolongs insulin half-life, heightening hypoglycemia risk. Dose reductions and close glucose monitoring are needed.
Pregnancy
Human insulin or well-studied analogs (e.g., Lispro, Aspart, Detemir) are generally safe. Intensive glucose control lowers the risk of fetal macrosomia or maternal complications.
Advances in Insulin Delivery Systems
Insulin Pumps
Real-time programmable pumps allow for basal rate adjustments, bolus wizards, and connectivity (some integrating CGM data for partial closed-loop solutions). They reduce glycemic variability and can improve quality of life in Type 1 patients.
Continuous Glucose Monitoring (CGM) Integration
Hybrid closed-loop or “artificial pancreas” systems combine a CGM sensor, an insulin pump, and an algorithm. They automatically modulate insulin delivery based on sensor glucose trends, significantly reducing hypo- and hyperglycemia episodes.
Inhaled Insulin
Technologies like Afrezza deliver rapid insulin powder to alveoli, where it is absorbed into circulation. While an appealing alternative to injections, concerns with respiratory function, absorption consistency, and patient acceptance limit adoption (Goodman & Gilman’s).
Implantable Pumps and Transplantation
Research extends to subcutaneous or intraperitoneal insulin pumps, islet cell transplantation, and stem-cell derived beta cells. However, these remain specialized or experimental.
Combination Therapies and Adjuncts
Amylin Analogs (Pramlintide)
Co-secreted with insulin from beta cells, amylin slows gastric emptying and reduces postprandial glucagon release. Injected as an adjunct to insulin, pramlintide helps reduce post-meal spikes. However, it requires separate injection.
GLP-1 Receptor Agonists
Agents like exenatide or liraglutide further enhance glucose-dependent insulin secretion, reduce glucagon, and slow gastric emptying. In Type 2 diabetes, they can be combined with basal insulin to improve control while avoiding weight gain.
SGLT2 Inhibitors
Though unrelated to insulin’s mechanism, these reduce renal glucose reabsorption. Pairing insulin with SGLT2 inhibitors might improve glycemia with less insulin needed. Still, caution for euglycemic ketoacidosis is warranted.
Optimizing Clinical Outcomes
Monitoring and Targets
Self-monitoring (fingerstick or CGM) plus periodic A1C checks guide adjustments. Targets may differ (e.g., <7% for most adults, individualized for older or comorbid patients). Menus of basal-bolus dosing, pump use, or once/twice-daily simplified regimens exist to balance tight control with risk of hypoglycemia.
Patient Education
Key instruction includes injection technique, recognition and treatment of hypoglycemia, dietary consistency, and safe travel measures. Frequent follow-up fosters adherence and timely adjustments (Katzung).
Risk-Benefit Considerations
Stricter control can reduce long-term microvascular (retinopathy, nephropathy, neuropathy) and some macrovascular complications. However, repeated severe hypoglycemia or excessive weight gain could offset benefits. Striking a balanced approach is essential for success.
Future Directions in Insulin Therapy
Ultra-Rapid Analog Developments
Formulations like faster-acting aspart (addition of niacinamide) shorten onset further, nearing physiologic first-phase insulin release. This may enhance postprandial glucose control with less late hypoglycemia.
Smart Insulin and Glucose-Responsive Systems
Research prototypes harness chemical or biologic gating, releasing insulin only when glucose surpasses a threshold. If commercialized, these might slash hypoglycemia risk while maintaining near-normal glycemia (Goodman & Gilman’s).
Gene Therapy and Regenerative Medicine
Investigational avenues include genetically modified non-beta cells producing insulin, or transplantable islets within protective capsules to shield from autoimmunity. While promising, such treatments remain far from routine clinical adoption.
Integration of AI in Insulin Dosing
Algorithms analyzing continuous data streams from CGM, activity, and meal detection could deliver fully autonomous closed-loop insulin infusion—an “artificial pancreas” capable of near physiologic control in real time.
Conclusion
Insulin stands as an irreplaceable hormone managing carbohydrate, protein, and lipid metabolism. Exogenous insulin therapy underpins the survival of Type 1 diabetes patients and significantly aids Type 2 cases requiring tighter glycemic control or advanced beta-cell failure. Progressively refined formulations—rapid, short, intermediate, and long-acting—as well as novel delivery technologies (pumps, CGM integration) allow increasingly precise replication of physiological insulin secretion. Nonetheless, the risk of hypoglycemia, weight gain, and injection burdens underscores the need for careful titration, education, and follow-up.
Ongoing research seeks to perfect insulin therapy’s convenience and safety—pursuing glucose-responsive insulins, advanced pump algorithms, and integrative closed-loop systems. From its discovery to its emerging frontiers, insulin exemplifies both a life-sustaining therapy and a model for translational science bridging endocrinology, pharmacology, and biotechnology. By optimizing insulin use in everyday practice—via personalized regimens, vigilant monitoring, and synergy with new agents—clinicians can help patients achieve stable euglycemia, mitigating diabetes complications and improving quality of life.
Book Citations
- Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 13th Edition.
- Katzung BG, Basic & Clinical Pharmacology, 15th Edition.
- Rang HP, Dale MM, Rang & Dale’s Pharmacology, 8th Edition.









[…] Insulin: A Comprehensive Guide […]