Metformin, a cornerstone in the treatment of type 2 diabetes mellitus, is one of the most commonly prescribed oral antidiabetic agents worldwide. Originally discovered from a natural plant source known as Galega officinalis (French lilac or goat’s rue), metformin has a long history of clinical use. Its popularity is largely due to its robust efficacy in lowering blood glucose, an acceptable side-effect profile, and well-studied outcomes. Beyond glycemic control, research has continuously uncovered a range of additional benefits, including cardiovascular protection and potential anti-cancer properties.
This comprehensive article explores the pharmacology of metformin by examining its history, classification, mechanism of action, pharmacodynamics, pharmacokinetics, clinical uses, side effects, adverse reactions, contraindications, drug interactions, special considerations, and future developments. While this information aims to be accurate and detailed, it should not be used as a substitute for personalized medical advice. Always consult a healthcare professional for individualized guidance.

Historical Background and Discovery
Ancient Roots
The history of metformin can be traced to the Middle Ages, when extracts from the Galega officinalis plant were used in Europe as a traditional remedy for symptoms resembling what we now characterize as diabetes. The plant’s active ingredient, guanidine, was identified in the late 19th century. However, guanidine itself was deemed too toxic for clinical use, prompting further derivative exploration.
Early 20th Century Developments
In the 1920s, alkylguanidines (including galegine) showed hypoglycemic properties in laboratory studies, but concerns about toxicity postponed their widespread therapeutic application. Other anti-hyperglycemic agents—such as insulin after its discovery in 1921—initially overshadowed any attempt to use guanidine-based compounds.
Reintroduction of Guanidine Derivatives
During the mid-20th century, French physician Jean Sterne conducted a clinical investigation on a compound called dimethylbiguanide, which we now know as metformin. By the 1950s, metformin proved its effectiveness in controlling hyperglycemia, eventually entering the market in Europe in the 1950s and 1960s. While other biguanides like phenformin and buformin were also developed, they exhibited a higher risk of lactic acidosis, leading to their withdrawal from many global markets.
Global Acceptance
Metformin’s major breakthrough in the United States occurred much later—approved by the FDA in 1994, it rapidly became a first-line therapy for type 2 diabetes. Today, metformin remains a universally recommended agent due to its reliable efficacy, generally good safety profile, and beneficial effects on overall morbidity and mortality in diabetic patients.
Classification of Metformin
Biguanides
Metformin belongs to the biguanide class of oral antihyperglycemic agents. The biguanide class is characterized by its ability to lower hepatic glucose output and improve insulin sensitivity in peripheral tissues. While phenformin and buformin were once used, they carried a higher propensity for adverse events, particularly lactic acidosis, leading to metformin’s ascendancy as the most commonly prescribed biguanide.
Alternative Classes of Oral Antidiabetics
Although the biguanides represent one class, there are several other categories of oral antidiabetic medications:
- Sulfonylureas (e.g., glipizide, glyburide)
- Thiazolidinediones (e.g., pioglitazone, rosiglitazone)
- DPP-4 Inhibitors (e.g., sitagliptin, vildagliptin)
- SGLT2 Inhibitors (e.g., canagliflozin, empagliflozin)
- Meglitinides (e.g., repaglinide)
- Alpha-glucosidase Inhibitors (e.g., acarbose, miglitol)
Among these, metformin stands out for its unique mechanism of action related to hepatic glucose production, its less frequent risk of hypoglycemia (when used as monotherapy), and its favorable weight profile.
Mechanism of Action
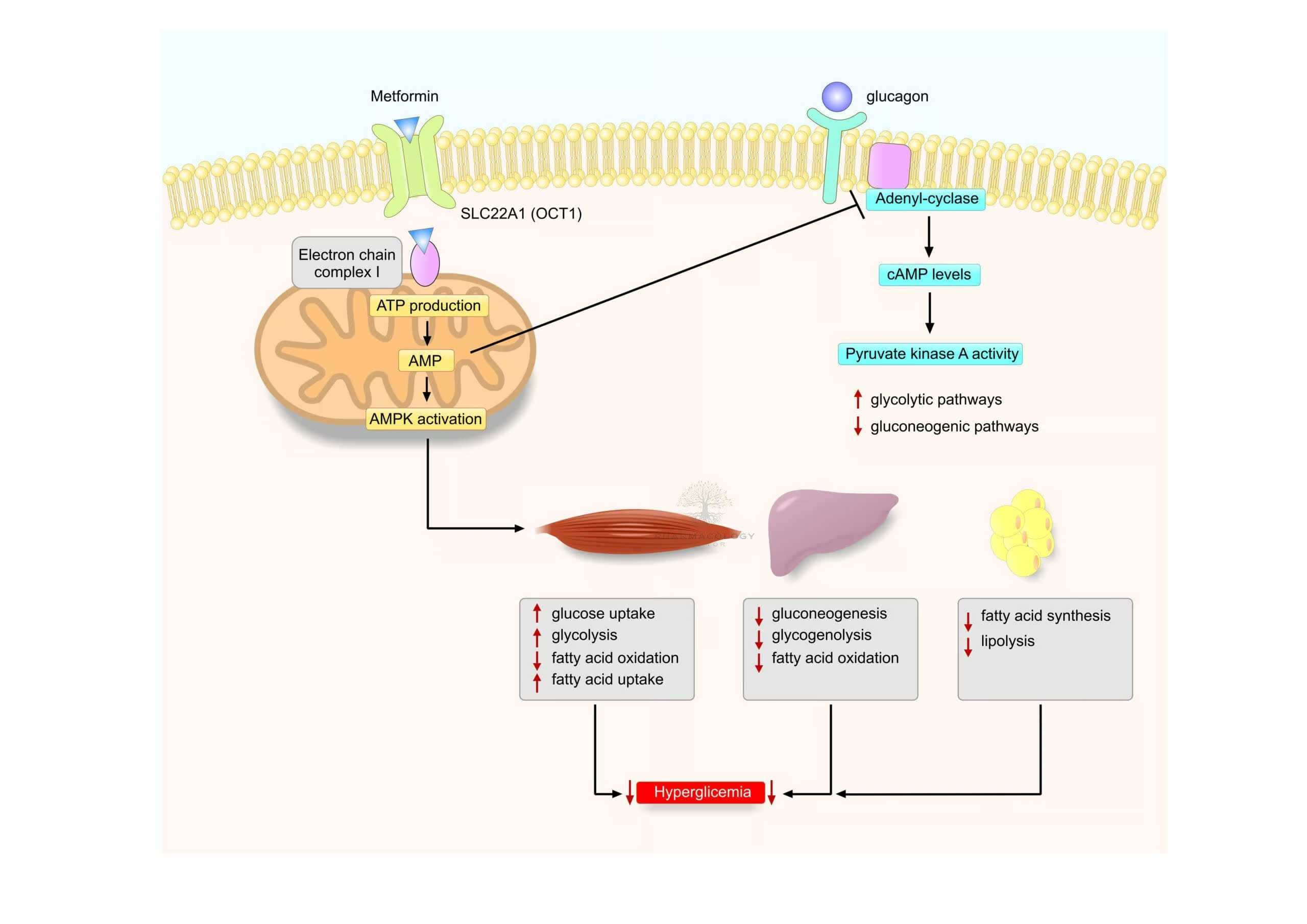
AMP-Activated Protein Kinase (AMPK) Activation
Central to metformin’s mechanism of action is the activation of AMP-activated protein kinase (AMPK). AMPK acts as a metabolic master switch, regulating cellular energy homeostasis. By activating AMPK, metformin decreases hepatic gluconeogenesis—the process by which the liver produces glucose. This effect lowers fasting plasma glucose levels and improves overall metabolic status in patients with type 2 diabetes.
Reduced Hepatic Glucose Output
The suppression of hepatic gluconeogenesis is widely regarded as metformin’s primary effect. It is achieved through multiple pathways, including inhibition of mitochondrial glycerophosphate dehydrogenase, thereby reducing the conversion of lactate and pyruvate into glucose. Additionally, by altering NAD+/NADH ratio, metformin modifies the hepatic redox state, further damping gluconeogenic flux.
Enhanced Peripheral Insulin Sensitivity
Metformin exerts a secondary effect of improving insulin sensitivity in peripheral tissues. It does so by increasing glucose uptake in skeletal muscle and adipose tissue. This effect, mediated in part by AMPK-related mechanisms, facilitates greater glucose clearance from the bloodstream with each insulin release.
Impact on the Gut
Studies have revealed that metformin’s actions are not limited to the liver and muscle. It also modifies glucose absorption and utilization in the intestines. Metformin may alter the microbiome, impacting the likelihood of improved glycemic control and possibly contributing to its mild weight-reducing or weight-neutral effects.
Effect on Lipid Metabolism
Although not always recognized as a primary target, metformin can reduce triglyceride levels and improve dyslipidemia, likely through its AMPK-mediated influence on fatty acid metabolism. This beneficial effect on lipid profiles may help reduce cardiovascular risks in diabetic patients.
Pharmacodynamics
Glycemic Control
Metformin consistently lowers fasting plasma glucose and hemoglobin A1c (HbA1c), often by 1–2 percentage points in patients newly diagnosed with type 2 diabetes. Due to its unique mechanism, the drug does not typically induce severe hypoglycemia when used as monotherapy, making it relatively safe compared to sulfonylureas and insulin (where exogenous sources can easily provoke low blood glucose).
Weight Neutral or Modestly Reductive
Unlike certain other antidiabetic medications like thiazolidinediones or insulin, metformin tends to be weight neutral or can even prompt modest weight loss over the long term, an advantage for many individuals with type 2 diabetes who may struggle with obesity.
Cardiovascular Benefits
Metformin gained significant attention when the UK Prospective Diabetes Study (UKPDS) suggested that patients on metformin might have reduced macrovascular complications, including myocardial infarction. While subsequent research has refined these findings, metformin is widely perceived as having a favorable or at least neutral effect on cardiovascular risk.
Impact on Other Systems
In addition to glucose metabolism and weight management, metformin may also affect hormonal pathways such as insulin-like growth factor (IGF) and androgen metabolism. These alterations can be beneficial in specific populations, such as women with polycystic ovary syndrome (PCOS), where metformin is frequently used to restore ovulatory cycles.
Pharmacokinetics
Absorption and Bioavailability
When administered orally, metformin is absorbed primarily in the small intestine. While the exact bioavailability can range from around 50% to 60%, the drug’s efficacy remains robust due to its high tissue uptake in the liver and muscles. Metformin’s absorption may be influenced by various patient-specific factors, including gastrointestinal motility and existing intestinal pathology.
Distribution
Metformin is not extensively bound to plasma proteins and displays a large volume of distribution. It accumulates primarily in the liver, gastrointestinal tract, and skeletal muscle, consistent with its clinical sites of action.
Metabolism
A hallmark of metformin is that it is not significantly metabolized by the liver. Instead, it primarily undergoes renal excretion. This feature lowers potential drug-drug interactions through metabolic pathways, although extrarenal clearance mechanisms exist on a smaller scale.
Excretion via Kidneys
Because the kidneys are the main route of excretion for metformin, patients with renal impairment face a heightened risk of drug accumulation, which can predispose them to lactic acidosis. Metformin is secreted unchanged in the urine, with a plasma elimination half-life typically around 4 to 9 hours in individuals with normal kidney function—some sources may indicate a half-life of up to 6.5 hours or more.
Clinical Uses
- First-Line Treatment for Type 2 Diabetes: Metformin is the gold standard initial therapy for most patients, barring specific contraindications such as advanced renal failure. The drug is often combined with lifestyle modifications (e.g., diet, exercise) to help manage hyperglycemia.
- Combination Therapy: When monotherapy with metformin fails to achieve glycemic targets, combination with other agents (like sulfonylureas, SGLT2 inhibitors, DPP-4 inhibitors, or injectable agents such as GLP-1 receptor agonists) can be highly effective.
- Prediabetes: In patients with impaired glucose tolerance, some guidelines recommend the use of metformin, particularly if the patient is overweight or has additional risk factors such as a history of gestational diabetes.
- Polycystic Ovary Syndrome (PCOS): Metformin sometimes helps restore regular menstrual cycles and ovulation in women with PCOS, also potentially aiding in weight management and insulin resistance.
- Other Potential Uses: Emerging research explores metformin’s potential roles in age-related conditions, cancer prevention, and cardiovascular disease modification. Although these indications remain under investigation, they highlight the broad interest in metformin’s metabolic effects.
Side Effects and Adverse Reactions
- Gastrointestinal Disturbances: Common complaints include nausea, diarrhea, abdominal bloating, and a metallic taste. These side effects may subside over time or be minimized by taking metformin with meals or using an extended-release (ER) formulation.
- Vitamin B12 Deficiency: Chronic metformin use can reduce vitamin B12 absorption, leading to potential deficiency and macrocytic anemia. Regular monitoring of B12 levels in long-term metformin users is advisable.
- Lactic Acidosis: Although rare, metformin-associated lactic acidosis (MALA) is a serious adverse effect that can be life-threatening. The incidence is especially higher in patients with renal insufficiency or other risk factors (e.g., acute heart failure, severe liver disease).
- Hypoglycemia (Rare in Monotherapy): Metformin, by itself, has a low propensity to cause significant hypoglycemia because it does not directly stimulate insulin secretion. However, the risk increases if it is combined with medications that do raise insulin levels, such as sulfonylureas or insulin itself.
- Skin Reactions: Though uncommon, some patients may report rashes or cutaneous hypersensitivity. These usually resolve upon discontinuing the medication or finding alternative therapies.
Contraindications and Precautions
- Severe Renal Impairment: Because metformin is renally excreted, patients with significantly reduced kidney function (e.g., an eGFR < 30 mL/min/1.73 m²) face higher lactic acidosis risk. Dose adjustments are advised if the eGFR is between 30-45 mL/min/1.73 m².
- Acute or Chronic Metabolic Acidosis: Conditions like diabetic ketoacidosis or lactic acidosis are absolute contraindications to metformin use until they have been corrected.
- Hypoperfusion States: Patients suffering from shock or severe respiratory conditions may not clear lactate adequately, thus increasing the probability of lactic acidosis.
- Chronic Liver Disease: Although mild to moderate hepatic impairment is no longer considered an absolute contraindication, assessment of individual risks is crucial.
- Before Iodinated Contrast Imaging: Patients are often advised to temporarily discontinue metformin before receiving iodinated contrast for imaging studies, especially if they have existing renal impairments.
Drug Interactions
Cimetidine
Cimetidine, an H2-receptor antagonist used for peptic ulcer disease, competes for renal tubular secretion. This interaction can raise plasma metformin levels by reducing its renal clearance. Though cimetidine is less frequently used nowadays due to the availability of alternative agents like ranitidine or proton pump inhibitors, the principle remains relevant for any drug that affects renal clearance.
Other Nephrotoxic Drugs
Nonsteroidal anti-inflammatory drugs (NSAIDs), certain diuretics, and other nephrotoxic agents can compromise renal function and thereby increase the risk of elevated metformin concentrations. Clinicians should be vigilant, especially in older patients or those with marginally acceptable renal function.
Alcohol
Excessive alcohol intake exacerbates the risk of lactic acidosis. Both metformin and ethanol can diminish lactate metabolism. Educating patients about responsible alcohol use or abstinence is strongly recommended.
Beta-Blockers
While not a direct alteration of metformin’s metabolism, beta-blockers can potentially mask some adrenergic symptoms of hypoglycemia. Patients on combination therapy (especially if also on sulfonylureas or insulin) should be educated to monitor blood glucose carefully.
Use in Special Populations
- Pediatric Patients: Metformin is approved for use in children with type 2 diabetes. Proper dose adjustments must be made according to the child’s weight and renal function, and GI side effects are monitored closely.
- Geriatric Population: Older adults have a higher probability of decreased renal function. Careful monitoring of eGFR ensures dosing remains safe. Additionally, older adults may be more susceptible to vitamin B12 deficiency.
- Pregnancy and Lactation: Metformin is classified under pregnancy category B in many regions, showing no evidence of harm in animal reproduction studies. It is commonly used for gestational diabetes and PCOS-related infertility. Although it is excreted in breast milk, levels to which an infant is exposed are generally minimal, and many clinicians consider it compatible with breastfeeding.
- Hepatic Impairment: Mild-to-moderate hepatic disease does not always preclude metformin use, but caution and close monitoring are necessary since hepatic impairment can alter lactate clearance.
Clinical Trials and Ongoing Research
Cardiovascular Outcomes Research
Metformin’s favorable cardiovascular profile has been backed by studies pointing to reductions in myocardial infarction risks and potential improvements in survival. Ongoing clinical trials aim to further elucidate these effects, focusing on whether early initiation of metformin in at-risk individuals could significantly cut down cardiovascular events.
Cancer Prevention and Treatment
Observational studies suggest a correlation between metformin use and reduced cancer incidence, particularly in colorectal, pancreatic, and breast cancers. There is active research investigating the molecular pathways—like AMPK activation and mTOR inhibition—through which metformin might influence cancer cell proliferation and metabolism. More randomized controlled trials will clarify its definitive role in cancer prophylaxis or adjunct therapy.
Alzheimer’s Disease and Neurodegeneration
Some preliminary research highlights metformin’s potential neuroprotective properties, hypothesizing that it alleviates insulin resistance in the brain and may help in diseases like Alzheimer’s. While still inconclusive, future studies are exploring whether improving metabolic function with metformin can slow cognitive decline.
Longevity and Anti-Aging
Metformin has attracted attention as a possible anti-aging medication. The Targeting Aging with Metformin (TAME) trial aims to determine if metformin can delay the onset of age-related ailments like cardiovascular disease, cancer, or dementia. While results are eagerly awaited, the pursuit of metformin for healthy aging remains speculative but of great interest.
Role in Type 2 Diabetes Mellitus
- First-Line Therapy: Whether used alone or in combination with other oral agents or insulin, metformin stands as the foundational therapy for T2DM. It is typically initiated as soon as the diagnosis is made in the absence of absolute or significant contraindications.
- Combination Therapy: When metformin monotherapy is insufficient for managing hyperglycemia, additional agents (like SGLT2 inhibitors or GLP-1 receptor agonists) can be added. Metformin usually remains part of the regimen due to its complementary mechanism of action and overall safety.
- Lifestyle Integration: A critical component of metformin therapy is the integration of diet and exercise modifications for optimizing glycemic control. Metformin’s effect on the body’s metabolism dovetails with lifestyle interventions to provide more robust improvements in insulin sensitivity.
Metformin in Polycystic Ovary Syndrome (PCOS)
Mechanisms in PCOS
Insulin resistance is a hallmark of PCOS. By improving insulin sensitivity, metformin helps decrease androgen production in the ovaries, offering beneficial effects on hyperandrogenism (e.g., hirsutism, acne). Additionally, lowered insulin resistance can restore normal menstrual cycles and ovulation, aiding fertility in PCOS patients.
Combined Lifestyle Approaches
Lifestyle interventions, such as weight loss and exercise, synergize effectively with metformin in PCOS management. This synergy can mitigate metabolic disturbances, reduce cardiovascular risks, and restore hormonal balance more effectively than either approach alone.
Fertility and Pregnancy
In some women struggling with infertility due to anovulation, metformin may improve ovulatory frequency. When combined with ovulation induction agents like clomiphene citrate, metformin can potentially enhance pregnancy rates. However, individualized assessment is crucial, given the complex hormonal interplay and varied clinical presentations in PCOS.
Cardiovascular Implications
- Protective Effects: Numerous observational and experimental studies highlight metformin’s ability to reduce cardiovascular mortality in type 2 diabetes patients. This protective effect arises partly from improved glycemic control, better lipid profiles, and reduced insulin resistance.
- Endothelial Function: Metformin may enhance endothelial function by reducing oxidative stress and inflammatory markers. These improvements can mitigate atherosclerotic plaque formation.
- Heart Failure Considerations: Although once considered relatively contraindicated in advanced heart failure due to concerns about tissue perfusion, more recent data suggest that metformin can be safe and beneficial if renal function is closely monitored, especially in stable heart failure patients.
Gastrointestinal Effects and Tolerability
Common GI Complaints
Nausea, diarrhea, and abdominal pain are some of the most frequently cited adverse effects. For many individuals, these side effects subside after the first few weeks of therapy if the dose is increased gradually.
Strategies to Improve GI Tolerance
Patients can mitigate GI discomfort by taking metformin with a meal or using extended-release formulations. Physicians may also suggest dose titration schedules, starting at a low dose (e.g., 500 mg once or twice daily) and then gradually increasing based on tolerance and glycemic response.
Role of Intestinal Microbiota
Recent research has uncovered changes in the composition of gut microbiota in patients on metformin. While still an emerging field, some scientists hypothesize that this shift in gut bacteria might partly contribute to both the beneficial metabolic effects and the GI side effects. Further exploratory work is expected to expand our understanding of the gut microbiome–metformin interaction.
Patient Counseling and Adherence
Education on Dose and Timing
Patients should be counseled to take metformin at the same time each day to ensure consistent plasma levels. Taking it with or shortly after meals can decrease GI side effects.
Encouraging Lifestyle Modifications
Because metformin’s full benefit materializes when combined with dietary measures and regular physical activity, providers should reinforce achievable lifestyle changes. Metformin can be a powerful motivator for patients to adopt healthier eating habits and exercise routines, as they often observe improvements in body weight and energy levels.
Monitoring for Nutritional Deficiencies
Given the risk of vitamin B12 deficiency with long-term use, routine checks can be considered, especially in patients with anemia, neuropathy, or other clinical manifestations suggestive of deficiency.
Future Perspectives
- Novel Formulations: Researchers are exploring improved formulations that could minimize GI side effects further, enhance bioavailability, and possibly reduce the dosing frequency to promote adherence.
- Biomarkers for Personalized Therapy: There is a growing interest in identifying genetic and metabolic biomarkers that predict which patients will respond optimally to metformin. Personalized medicine in diabetes care could improve outcomes and reduce trial-and-error approaches.
- Combining Agents: The future might bring an expanded role of dual-therapy or triple-therapy combinations started from the outset of T2DM treatment, ensuring earlier glycemic control and addressing multiple facets of metabolic syndrome simultaneously.
- Expanding Indications: As trials on anti-aging, cancer prophylaxis, and neuroprotection continue, metformin’s role may extend well beyond its current scope in metabolic disorders.
Practical Tips for Clinicians
- Start Low and Go Slow: When initiating metformin, a common approach is to start at 500 mg once or twice daily and increase gradually to the target dose (often 2000 mg/day, split across 2-3 doses, or equivalent extended-release dosing) to minimize GI side effects.
- Assess Renal Function: Always review eGFR prior to starting metformin and periodically thereafter. Adjust doses or discontinue based on established clinical guidelines if renal function declines.
- Warn About Diet and Alcohol: Encourage patients to avoid excessive alcohol intake, which can raise lactic acidosis risk. Emphasize that dietary choices can vastly improve glycemic control and synergy with metformin.
- Monitor B12 Levels: In long-term metformin users, especially those with suggestive symptoms (anemia, neuropathy), consider periodic vitamin B12 level checks.
- Patient Engagement: Educate patients on potential GI side effects, reassure them these may improve over time, and stress the significance of regimen adherence for optimal results.
Conclusion
Metformin remains a central pillar in the pharmacological arsenal for type 2 diabetes management, supported by robust evidence of efficacy, cardiovascular benefits, and a generally favorable safety profile. Its unique mechanisms—centered around AMPK activation, inhibition of hepatic gluconeogenesis, and improvement of peripheral insulin sensitivity—have set it apart from other glucose-lowering agents. Moreover, its potential roles in PCOS, gestational diabetes, cancer prevention, and longevity research speak to its wide-reaching influence on modern medicine.
Despite facing some limitations, such as gastrointestinal side effects and rare but notable risks like lactic acidosis, metformin’s benefits typically outweigh these concerns when used judiciously. Proper patient selection, dose titration, and regular monitoring of renal and nutritional parameters ensure safety and efficacy. As the medical community continues to explore metformin’s wide-ranging effects—spanning oncology, cardiology, and beyond—this humble biguanide stands poised to remain a foundational therapy in metabolic health for years to come.
Ultimately, metformin’s story is testament to how deep inquiry—warrior-like persistence through centuries of herbal traditions and modern-day research—can bring about a transformative therapy that addresses one of the most pressing public health challenges: type 2 diabetes. Its position as the gold standard in T2DM treatment is well-earned, and ongoing studies may continue to expand our knowledge, improving the care of countless individuals around the globe.









[…] Biguanides – Metformin […]