1. Introduction to Diabetes Mellitus and Glycemic Control
1.1. Overview of Diabetes Mellitus
Diabetes Mellitus (DM) is not a single entity but a heterogeneous group of metabolic disorders characterised by chronic hyperglycemia. This elevated blood glucose results from defects in insulin secretion, insulin action, or, most commonly, both. The chronic nature of this hyperglycemia is associated with significant long-term damage, dysfunction, and failure of various organs, especially the eyes (retinopathy), kidneys (nephropathy), nerves (neuropathy), heart (cardiovascular disease), and blood vessels.
The global prevalence of DM is rising at an alarming rate, making it a major public health crisis. The classification of DM includes several types, but the two most prevalent forms are:
- Type 1 Diabetes Mellitus (T1DM): This form, typically accounting for 5-10% of cases, is characterized by an absolute deficiency of insulin secretion. It is an autoimmune disease in which the body’s immune system, mediated by T-cells, attacks and destroys the insulin-producing beta cells (β-cells) in the pancreatic islets of Langerhans. Patients with T1DM require exogenous insulin for survival.
- Type 2 Diabetes Mellitus (T2DM): This form accounts for over 90% of all diabetes cases. It is characterized by a combination of two primary pathophysiological defects:
- Insulin Resistance: A decreased sensitivity of peripheral target tissues (primarily liver, skeletal muscle, and adipose tissue) to the actions of insulin.
- Beta-Cell Dysfunction: A progressive inability of the pancreatic β-cells to compensate for this resistance by secreting adequate amounts of insulin.
1.2. Pathophysiology as a Target for Pharmacotherapy
Understanding the complex pathophysiology of T2DM is essential for rational pharmacotherapy. The “Ominous Octet,” proposed by DeFronzo, outlines eight distinct pathophysiological defects that contribute to hyperglycemia in T2DM, providing a conceptual framework for the targets of modern antidiabetic agents:
- Pancreatic β-cell: Impaired insulin secretion.
- Pancreatic α-cell: Excess glucagon secretion.
- Liver: Increased hepatic glucose production (gluconeogenesis and glycogenolysis).
- Skeletal Muscle: Decreased peripheral glucose uptake.
- Adipose Tissue: Increased lipolysis and associated insulin resistance.
- Kidney: Increased renal glucose reabsorption.
- Gastrointestinal Tract: Diminished “incretin effect.”
- Brain: Neurotransmitter dysfunction and central insulin resistance.
1.3. Therapeutic Goals
The primary goal of antidiabetic therapy is to achieve and maintain optimal glycemic control, thereby preventing acute complications (e.g., diabetic ketoacidosis, hyperosmolar hyperglycemic state) and reducing the risk of long-term microvascular and macrovascular complications.
- Glycemic Targets: These are assessed by measuring:
- Glycated Hemoglobin (HbA1c): Reflects mean blood glucose over the preceding 2-3 months.
- Fasting Plasma Glucose (FPG): Glucose level after an 8-hour fast.
- Postprandial Glucose (PPG): Glucose level 1-2 hours after a meal.
- Beyond Glycemia: Modern therapeutic strategies have evolved significantly. The goal is no longer just “sugar control.” Landmark clinical trials have demonstrated that certain drug classes offer profound cardiovascular (CV) and renal protection, independent of their glucose-lowering effects. Therefore, therapeutic choices are now heavily influenced by the presence of comorbidities such as atherosclerotic cardiovascular disease (ASCVD), heart failure (HF), and chronic kidney disease (CKD).
This chapter will review the pharmacokinetics, pharmacodynamics, mechanisms of action, clinical uses, and adverse effects of the major classes of antidiabetic drugs.
2. Insulin Preparations
Insulin is the cornerstone of therapy for all patients with T1DM and for many patients with advanced T2DM who fail to achieve glycemic goals with non-insulin agents.
2.1. Physiology of Insulin
Endogenous insulin is a 51-amino-acid polypeptide synthesized in the pancreatic β-cell as a precursor, proinsulin. Proinsulin is cleaved to form active insulin and C-peptide, which are co-secreted in equimolar amounts. Insulin secretion is primarily triggered by elevated blood glucose, which enters the β-cell via the GLUT2 transporter, is metabolized to produce ATP, and closes the ATP-sensitive potassium (K-ATP) channel. This depolarizes the cell membrane, opening voltage-gated calcium channels, and the subsequent influx of Ca²⁺ triggers the exocytosis of insulin-containing granules.
In target tissues, insulin binds to the insulin receptor (IR), a tyrosine kinase receptor. This binding initiates a complex intracellular signaling cascade (e.g., via IRS proteins, PI3K/Akt pathway, and MAPK pathway) that ultimately promotes the translocation of GLUT4 (glucose transporter 4) to the cell membrane in muscle and adipose tissue, facilitating glucose uptake. In the liver, insulin suppresses gluconeogenesis and glycogenolysis while promoting glycogen synthesis.
2.2. Pharmacokinetics of Insulin
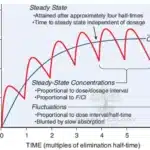
Exogenous insulin is a protein and is therefore degraded in the gastrointestinal tract if taken orally. It must be administered parenterally, most commonly via subcutaneous (SC) injection. The rate of absorption from the SC site is the primary determinant of its onset and duration of action. Regular human insulin, when injected subcutaneously, self-associates into hexamers (stabilized by zinc), which must first dissociate into dimers and then monomers to be absorbed into the bloodstream. This dissociation process creates a lag in onset and a prolonged duration that does not mimic natural physiologic insulin release.
Modern insulin analogs were engineered by modifying the amino acid sequence of human insulin to alter these aggregation properties, thereby creating more predictable and physiologically appropriate pharmacokinetic (PK) profiles.
2.3. Classification of Insulin Preparations
Insulin preparations are classified based on their onset, peak, and duration of action.
2.3.1. Rapid-Acting Analogs
- Drugs: Insulin Lispro (Lys-Pro reversed), Insulin Aspart (Proline to Aspartic acid), Insulin Glulisine (Lysine to Glutamic acid).
- Pharmacokinetics: These analogs have reduced self-aggregation potential. They exist primarily as monomers in solution and after injection, allowing for very rapid absorption, onset (10-20 minutes), peak (1-1.5 hours), and short duration (3-5 hours).
- Clinical Use: Primarily used as prandial (bolus) insulin to control post-meal glucose excursions. They should be injected 0-15 minutes before a meal. They are also the preferred insulin for use in continuous subcutaneous insulin infusion (CSII) pumps.
2.3.2. Short-Acting Insulin
- Drug: Regular (Human) Insulin
- Pharmacokinetics: This is the native human insulin sequence produced by recombinant DNA technology. Its hexameric structure results in a slower onset (30-60 minutes), later peak (2-4 hours), and longer duration (6-8 hours) compared to rapid-acting analogs.
- Clinical Use: Used for prandial coverage, but its PK profile is suboptimal, requiring injection 30-45 minutes before a meal. It is the only insulin formulation suitable for intravenous (IV) administration (e.g., in the management of Diabetic Ketoacidosis (DKA) or during critical illness).
2.3.3. Intermediate-Acting Insulin
- Drug: NPH (Neutral Protamine Hagedorn) Insulin
- Pharmacokinetics: NPH is a suspension of regular insulin complexed with protamine and zinc. The protamine must be enzymatically cleaved at the injection site, slowing the release and absorption of the insulin. This results in an onset of 2-4 hours, a peak at 4-10 hours, and a duration of 12-18 hours.
- Clinical Use: Provides basal insulin coverage. Its pronounced peak, high variability, and non-physiologic duration have led to its replacement by long-acting analogs in many settings, though it remains a lower-cost option.
2.3.4. Long-Acting (Basal) Analogs
These analogs are designed to provide a steady, “peakless” basal level of insulin over 24 hours.
- Insulin Glargine (U-100 and U-300):
- Mechanism: Glargine is soluble at an acidic pH (in the vial) but precipitates in the neutral pH of the subcutaneous tissue after injection. This micro-precipitate forms a depot from which insulin monomers are slowly and continuously released.
- PK: Onset is 1-2 hours. Glargine U-100 has a duration of ~24 hours and is largely peakless. Glargine U-300 (Toujeo) is a more concentrated form (300 U/mL) that forms a more compact depot, resulting in an even flatter and longer duration (>24 hours), with a lower risk of hypoglycemia compared to U-100.
- Insulin Detemir:
- Mechanism: A myristic acid (a fatty acid) chain is acylated to the B-chain. This modification promotes (1) self-aggregation at the injection site and (2) reversible binding to serum albumin in the bloodstream, which serves as a circulating reservoir.
- PK: Onset is 1-2 hours. Duration is dose-dependent, lasting 12-24 hours. It often requires twice-daily (BID) dosing for full basal coverage.
- Insulin Degludec:
- Mechanism: This “ultra-long-acting” analog forms stable, soluble multi-hexamer chains in the subcutaneous depot. Insulin monomers slowly dissociate from the ends of these chains.
- PK: Has the flattest and most predictable profile with a duration of action >42 hours. This allows for more flexible dosing (though daily dosing is still recommended) and is associated with the lowest rates of nocturnal hypoglycemia.
2.4. Therapeutic Use and Adverse Effects
- Clinical Use:
- T1DM: Requires a basal-bolus regimen (a long-acting analog for basal needs + a rapid-acting analog for prandial/correction needs) or CSII pump therapy.
- T2DM: Added when non-insulin agents fail to maintain glycemic targets, often starting with a single dose of basal insulin.
- Emergencies: IV Regular insulin is used for DKA and Hyperosmolar Hyperglycemic State (HHS).
- Adverse Effects:
- Hypoglycemia: The most common and serious adverse effect. It is a direct consequence of insulin’s pharmacologic action. Symptoms include tremor, tachycardia, sweating (autonomic) and confusion, coma, seizures (neuroglycopenic).
- Weight Gain: A common side effect, due to the anabolic effects of insulin and a reduction in glucosuria.
- Lipodystrophy: Atrophy or hypertrophy of subcutaneous fat at injection sites. Lipohypertrophy is more common and results from the lipogenic effect of high local insulin concentrations, which can impair insulin absorption. This is prevented by rotating injection sites.
- Hypersensitivity: Rare, can be local or systemic.
3. Non-Insulin Antidiabetic Agents
These agents, primarily used for T2DM, target the various pathophysiological defects of the disease.
3.1. Agents Increasing Insulin Sensitivity
These drugs improve the body’s response to its own insulin.
3.1.1. Biguanides
- Drug: Metformin
- Mechanism of Action: Metformin is the undisputed first-line agent for T2DM. Its mechanism is complex and not fully elucidated, but its primary effect is the reduction of hepatic glucose production (gluconeogenesis). It achieves this by inhibiting mitochondrial complex I, which increases cellular AMP/ATP ratios and activates AMP-activated protein kinase (AMPK). AMPK activation has downstream effects including suppression of gluconeogenic gene expression and increased glucose uptake in skeletal muscle.
- Pharmacokinetics: Administered orally. It is not protein-bound and is not metabolized. It is excreted unchanged by the kidneys via active tubular secretion.
- Clinical Effects: Lowers FPG significantly. It is euglycemic, meaning it does not cause hypoglycemia when used as monotherapy. It is weight-neutral or associated with modest weight loss. It also has known cardiovascular benefits.
- Adverse Effects:
- Gastrointestinal Distress: Very common (up to 30%). Includes diarrhea, nausea, abdominal cramping, and a metallic taste. Usually dose-dependent and can be minimized by starting at a low dose, titrating slowly, and taking it with food. Extended-release (XR) formulations are better tolerated.
- Lactic Acidosis: A rare (<1 in 30,000) but life-threatening complication. Metformin can inhibit the hepatic metabolism of lactic acid. The risk is virtually zero in patients with normal renal function but is significantly increased in states of renal impairment, tissue hypoxia (e.t., severe HF, sepsis), or acute liver failure.
- Vitamin B12 Deficiency: Chronic use can interfere with B12 absorption in the terminal ileum.
- Contraindications: Severely reduced renal function (eGFR < 30 mL/min/1.73m²), acute/unstable heart failure, severe liver disease, and acute metabolic acidosis.
3.1.2. Thiazolidinediones (TZDs or “Glitazones”)
- Drugs: Pioglitazone, Rosiglitazone (rarely used)
- Mechanism of Action: TZDs are selective agonists for the Peroxisome Proliferator-Activated Receptor-gamma (PPAR-γ), a nuclear receptor. PPAR-γ is highly expressed in adipose tissue. Activation of this receptor alters the transcription of numerous genes involved in glucose and lipid metabolism. The primary effect is to increase insulin sensitivity in peripheral tissues (adipose, muscle, liver). TZDs promote the differentiation of pre-adipocytes into mature fat cells that are more “insulin-sensitive” and store fatty acids more effectively, thereby reducing lipotoxicity in other organs (re-partitioning of fat). This is a slow genomic effect; maximal glucose-lowering may take 3-4 months.
- Clinical Effects: Potent glucose-lowering. Can be used as monotherapy or in combination. Does not cause hypoglycemia as monotherapy.
- Adverse Effects:
- Weight Gain: Common, due to both fluid retention and an increase in subcutaneous adipose mass.
- Edema/Fluid Retention: TZDs can cause peripheral edema and are strongly contraindicated in patients with New York Heart Association (NYHA) Class III or IV heart failure.
- Bone Fractures: Increased risk of fractures, particularly in the distal limbs of postmenopausal women, dueto effects on bone marrow stem cell differentiation (favoring adipocytes over osteoblasts).
- Bladder Cancer: A small, controversial increased risk associated with Pioglitazone.
- (Historical: Rosiglitazone was previously restricted due to concerns of increased myocardial infarction risk, but subsequent large trials (e.g., RECORD) did not confirm this. It remains rarely used.)
3.2. Agents Enhancing Insulin Secretion (Secretagogues)
These drugs stimulate the pancreas to release more insulin, regardless of the ambient glucose level.
3.2.1. Sulfonylureas (SUs)
- Drugs:
- First-generation (historical, rarely used): Tolbutamide, Chlorpropamide
- Second-generation (current): Glyburide (Glibenclamide), Glipizide, Glimepiride, Gliclazide
- Mechanism of Action: SUs bind to the sulfonylurea receptor (SUR1), which is a regulatory subunit of the K-ATP channel on the pancreatic β-cell membrane. This binding closes the K-ATP channel, preventing potassium efflux. This leads to depolarization of the cell membrane, which opens voltage-gated Ca²⁺ channels. The resulting influx of Ca²⁺ triggers the exocytosis and release of pre-formed insulin.
- Pharmacokinetics: Well-absorbed orally. Metabolized by the liver (CYP450). Glyburide has an active metabolite that is renally cleared, making it a poor choice in renal impairment.
- Clinical Effects: Effective at lowering both FPG and PPG.
- Adverse Effects:
- Hypoglycemia: The most significant and dangerous side effect. Because their action is glucose-independent, they can continue to drive insulin secretion even when glucose is low. The risk is higher with long-acting agents (Glyburide) and in elderly or renally-impaired patients.
- Weight Gain: Common, due to the anabolic effects of increased insulin levels.
- Other: Skin rash, nausea. The efficacy of SUs wanes over time (“secondary failure”) as β-cell function progressively declines.
3.2.2. Meglitinides (Glinides)
- Drugs: Repaglinide, Nateglinide
- Mechanism of Action: Similar to SUs, meglitinides bind to the β-cell and close the K-ATP channel (at a different binding site than SUs). However, they have a very rapid onset and a very short duration of action.
- Clinical Effects: Their PK profile makes them ideal for prandial glucose control. They are taken just before meals (“take with meals, skip if meal is skipped”) to reduce post-meal glucose spikes.
- Adverse Effects:
- Hypoglycemia: Risk is present but is generally lower and less prolonged than with SUs due to the short duration of action.
- Weight Gain: Possible, but often less than with SUs.
3.3. Incretin-Based Therapies
This class of drugs leverages the “incretin effect.” Incretins are gut-derived hormones (e.g., Glucagon-Like Peptide-1 (GLP-1) and Glucose-dependent Insulinotropic Polypeptide (GIP)) that are released in response to nutrient ingestion. They potentiate insulin secretion in a glucose-dependent manner (i.e., only when blood glucose is high), suppress glucagon secretion, slow gastric emptying, and promote satiety. In T2DM, this effect is blunted.
3.3.1. GLP-1 Receptor Agonists (GLP-1 RAs)
- Drugs: These are injectable peptides, classified by duration.
- Short-acting (prandial focus): Exenatide (BID), Liraglutide (QD)
- Long-acting (basal focus): Exenatide XR (QW), Dulaglutide (QW), Semaglutide (QW)
- Oral: Oral Semaglutide (the first peptide available in an oral form)
- Mechanism of Action: These drugs are analogs of human GLP-1 that are resistant to degradation by the enzyme Dipeptidyl Peptidase-4 (DPP-4). They bind to and activate the GLP-1 receptor, resulting in:
- Glucose-dependent insulin release (potent).
- Suppression of inappropriately high glucagon secretion.
- Slowing of gastric emptying.
- Significant central effects on the hypothalamus, leading to increased satiety and reduced appetite.
- Clinical Effects:
- Potent A1c reduction.
- Significant Weight Loss: A major advantage, making them ideal for overweight/obese patients.
- Cardiovascular Benefit: Several (Liraglutide, Semaglutide, Dulaglutide) have shown in large CV outcome trials (CVOTs) to reduce rates of Major Adverse Cardiovascular Events (MACE), particularly in patients with established ASCVD.
- Low Hypoglycemia Risk: Action is glucose-dependent.
- Adverse Effects:
- Gastrointestinal: Nausea, vomiting, and diarrhea are very common, especially upon initiation. This is due to the gastric-slowing and central effects. Usually, this is transient and resolves by titrating the dose slowly.
- Pancreatitis: A rare risk; cases of acute pancreatitis have been reported.
- Thyroid C-Cell Tumors: A boxed warning based on rodent studies (medullary thyroid carcinoma). This has not been established in humans, but they are contraindicated in patients with a personal or family history of MTC or Multiple Endocrine Neoplasia (MEN) type 2.
3.3.2. DPP-4 Inhibitors (“Gliptins”)
- Drugs: Sitagliptin, Saxagliptin, Linagliptin, Alogliptin
- Mechanism of Action: These are oral small molecules that inhibit the DPP-4 enzyme. DPP-4 is responsible for the rapid inactivation (within 1-2 minutes) of endogenous incretins (GLP-1 and GIP). By inhibiting DPP-4, these drugs prolong the half-life and increase the concentrations of the body’s own incretins, thereby enhancing their physiologic effects (glucose-dependent insulin release, glucagon suppression).
- Clinical Effects: Modest A1c reduction. Weight-neutral. Extremely well-tolerated. Low risk of hypoglycemia.
- Adverse Effects:
- Generally very few, which is their main advantage.
- Pancreatitis: Rare reports, similar to GLP-1 RAs.
- Joint Pain: Severe arthralgia has been reported, requiring discontinuation.
- Heart Failure Risk: Saxagliptin and Alogliptin were associated with a small increased risk of hospitalization for heart failure in CVOTs (SAVOR-TIMI 53, EXAMINE).
- Pharmacokinetics (Notable): Most are renally dosed, except for Linagliptin, which is excreted via the bile and gut, making it a safe and simple option for patients with CKD.
3.4. Agents Increasing Urinary Glucose Excretion
3.4.1. SGLT2 Inhibitors (“Gliflozins”)
- Drugs: Canagliflozin, Dapagliflozin, Empagliflozin, Ertugliflozin
- Mechanism of Action: This class provides an entirely insulin-independent mechanism. The kidneys filter ~180 grams of glucose per day, and >90% of this is reabsorbed in the proximal convoluted tubule by the Sodium-Glucose Co-transporter 2 (SGLT2). These drugs selectively inhibit SGLT2, blocking the reabsorption of glucose. This results in the excretion of excess glucose in the urine (glucosuria), thereby lowering blood glucose.
- Clinical Effects:
- Modest A1c reduction.
- Weight Loss: Due to the caloric loss (glucosuria).
- Blood Pressure Reduction: A mild osmotic diuretic effect.
- Cardio-Renal Protection: This is the most profound benefit of the class. Multiple CVOTs (EMPA-REG OUTCOME, CANVAS, DECLARE-TIMI 58) have demonstrated that Empagliflozin, Canagliflozin, and Dapagliflozin:
- Reduce MACE in patients with established ASCVD.
- Significantly reduce hospitalization for heart failure (even in patients without diabetes).
- Provide robust renal protection, slowing the progression of CKD.
- Low Hypoglycemia Risk: Action is insulin-independent.
- Adverse Effects:
- Genital Mycotic Infections: (Yeast infections). The most common side effect (~10% of females, ~4% of males), due to the high glucose content in the urine.
- Urinary Tract Infections (UTIs): A small increased risk.
- Volume Depletion/Hypotension: Due to the osmotic diuretic effect. Caution in the elderly or those on diuretics.
- Euglycemic Diabetic Ketoacidosis (eDKA): A rare but serious complication. DKA occurs but with only modestly elevated blood glucose (<250 mg/dL), making diagnosis difficult. Risk is higher in T1DM (off-label use), during illness, or with very low carbohydrate intake.
- Fractures / Amputation Risk: An increased risk of bone fractures and (in the CANVAS trial) lower-limb amputations was seen with Canagliflozin, though the mechanism is unclear and may not be a class effect.
3.5. Agents Affecting Glucose Absorption
3.5.1. Alpha-Glucosidase Inhibitors
- Drugs: Acarbose, Miglitol
- Mechanism of Action: These drugs competitively inhibit alpha-glucosidase enzymes (e.g., maltase, sucrase) located in the brush border of the small intestine. These enzymes are responsible for breaking down complex carbohydrates into absorbable monosaccharides (like glucose). By inhibiting them, the drugs delay carbohydrate digestion and absorption, thereby reducing the magnitude of postprandial glucose excursions.
- Clinical Effects: Modest A1c reduction, primarily targets PPG. Does not cause hypoglycemia as monotherapy.
- Adverse Effects:
- Gastrointestinal: Extremely common and prominent. Includes flatulence, diarrhea, and abdominal cramping. This is caused by the undigested carbohydrates reaching the colon, where they are fermented by bacteria. This side effect profile leads to very poor tolerability and adherence.
3.6. Other Agents
- Amylin Analogs:
- Drug: Pramlintide (Injectable)
- Mechanism: Pramlintide is a synthetic analog of amylin, a peptide hormone co-secreted with insulin from β-cells. Amylin complements insulin’s actions by (1) slowing gastric emptying, (2) suppressing postprandial glucagon secretion, and (3) promoting satiety.
- Use: An adjunctive therapy for T1DM and T2DM patients who use prandial insulin but have failed to achieve target glucose.
- Adverse Effects: Nausea (very common) and a high risk of hypoglycemia when used with insulin (insulin dose must be reduced).
4. Therapeutic Strategies and Future Directions
The management of T2DM has shifted from a “glycemic-centric” to a “comorbidity-centric” approach.
- Monotherapy: Metformin remains the first-line agent for almost all patients with T2DM, given its efficacy, safety, low cost, and CV benefits.
- Combination Therapy: If Metformin monotherapy is insufficient, the choice of a second agent is guided by patient-specific factors:
- Patient with ASCVD, HF, or CKD: An SGLT2 inhibitor or a GLP-1 receptor agonist with proven cardio-renal benefit is strongly recommended, regardless of the A1c level.
- Need for maximum efficacy / Weight Loss: A GLP-1 RA is preferred.
- Goal to minimize hypoglycemia: SGLT2i, GLP-1 RA, DPP-4i, or TZD are preferred over SUs.
- Cost is a major barrier: A Sulfonylurea or TZD may be considered.
Future Directions:
Pharmacology continues to evolve. Dual-agonist therapies, such as Tirzepatide (a GIP/GLP-1 receptor co-agonist), have shown even greater efficacy in A1c reduction and weight loss than GLP-1 RAs alone. Research is also focused on oral peptide formulations, novel insulin-sensitizers, and agents that can preserve or restore β-cell mass and function.
5. Conclusion
The pharmacologic armamentarium for diabetes is vast and targets nearly every aspect of its complex pathophysiology. From the life-saving replacement of insulin in T1DM to the sophisticated, multi-faceted approach to T2DM, these agents are critical tools. The modern prescriber must not only aim for glycemic targets but must also synthesize a comprehensive treatment plan that addresses a patient’s cardiovascular and renal risk, a paradigm shift driven by the powerful clinical trial evidence for the SGLT2 inhibitor and GLP-1 receptor agonist classes.
📚 AI Pharma Quiz Generator
🎉 Quiz Results
Medical Disclaimer
The medical information on this post is for general educational purposes only and is provided by Pharmacology Mentor. While we strive to keep content current and accurate, Pharmacology Mentor makes no representations or warranties, express or implied, regarding the completeness, accuracy, reliability, suitability, or availability of the post, the website, or any information, products, services, or related graphics for any purpose. This content is not a substitute for professional medical advice, diagnosis, or treatment; always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition and never disregard or delay seeking professional advice because of something you have read here. Reliance on any information provided is solely at your own risk.