Introduction
Nausea and vomiting are protective reflexes designed to prevent the ingestion or continued digestion of harmful toxins. However, when severe, they can significantly deteriorate patient comfort, compromise treatment adherence, and lead to complications such as electrolyte imbalances, dehydration, and undernutrition. Antiemetic drugs play a critical role across various clinical contexts—perioperative care, chemotherapy, radiation therapy, infectious gastroenteritis, and motion sickness—to mitigate or prevent episodes of emesis. Over the past few decades, the understanding of the neurophysiological pathways of nausea and vomiting has grown extensively, leading to more refined and targeted antiemetic strategies.
Therapeutic choices incorporate agents targeting different receptor mechanisms and physiological pathways, including dopaminergic (D₂) blockade, serotonin (5-HT₃) antagonism, neurokinin-1 (NK₁) antagonism, muscarinic blockade, histamine-1 (H₁) blockade, modulation of the chemoreceptor trigger zone (CTZ), and central sedation. By systematically analyzing the pharmacology, effectiveness, and side effect profiles of these antiemetic agents, we can tailor regimens to various etiologies of nausea and vomiting.
In this comprehensive review, we will delve into the pharmacology of antiemetic drugs, referencing leading standard texts like Goodman & Gilman’s The Pharmacological Basis of Therapeutics (13th Edition), Katzung BG, Basic & Clinical Pharmacology (15th Edition), and Rang & Dale’s Pharmacology (8th Edition).
Physiology of Nausea and Vomiting
The Vomiting Center and Afferent Pathways
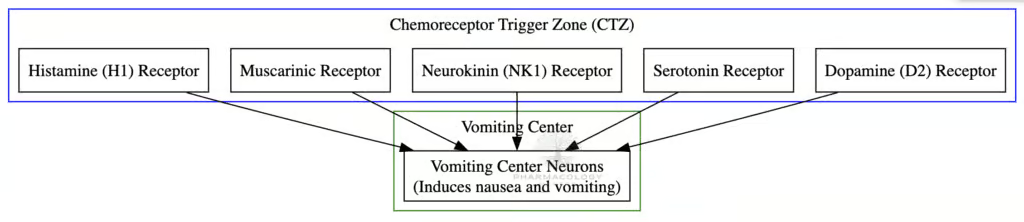
Nausea and vomiting originate from a coordinated response involving peripheral and central stimuli converging on the vomiting center in the medulla oblongata. Key participants include:
- Chemoreceptor Trigger Zone (CTZ): Located in the area postrema of the medulla. It is highly sensitive to emetic drugs and toxins due to its relatively permeable blood-brain barrier. The CTZ harbors multiple receptors—dopamine D₂, serotonin 5-HT₃, and neurokinin-1—that, when stimulated, transmit impulses to the vomiting center.
- Vestibular System: Equipped with muscarinic (M1) and histaminic (H1) receptors. This pathway contributes to motion sickness, as aberrant vestibular input can trigger emesis.
- Visceral Afferents (Vagal and Splanchnic): Irritation of the gastrointestinal (GI) tract—by toxins, distension, chemotherapy—induces the release of serotonin (5-HT) from enterochromaffin cells. This stimulates 5-HT₃ receptors on vagal afferents, sending signals to the nucleus tractus solitarius (NTS).
- Higher Centers (Cortex, Limbic System): Anxiety, stress, or certain sensory stimuli can initiate a vomiting reflex via cortical-limbic modulation of the medulla.
The vomiting center integrates these signals and coordinates the motor activity needed for retching and emesis, guiding the diaphragm, abdominal muscles, and esophagus to expel gastric contents.
Key Neurotransmitters in Emesis
Multiple neurotransmitters mediate the emetic response:
- Dopamine (D₂): Central to the CTZ’s response to systemic toxins or drugs.
- Serotonin (5-HT₃): Released from enterochromaffin cells in the GI tract upon exposure to noxious stimuli, prominently driving chemotherapy-induced nausea and vomiting (CINV).
- Neurokinin-1 (NK₁): Substance P acts on NK₁ receptors in the vomiting center and CTZ, complementing 5-HT₃ in generating both acute and delayed emesis.
- Acetylcholine (Muscarinic M1): Mediates motion sickness and labyrinthine stimulation.
- Histamine (H1): Also strongly associated with motion sickness.
- Gamma-Aminobutyric Acid (GABA): While not a primary driver of emesis, its modulation can help reduce anxiety-mediated anticipatory vomiting (e.g., via benzodiazepines).
By understanding each transmitter’s role, clinicians can adopt a multi-target approach, using numerous antiemetic classes in synergy to optimize control.
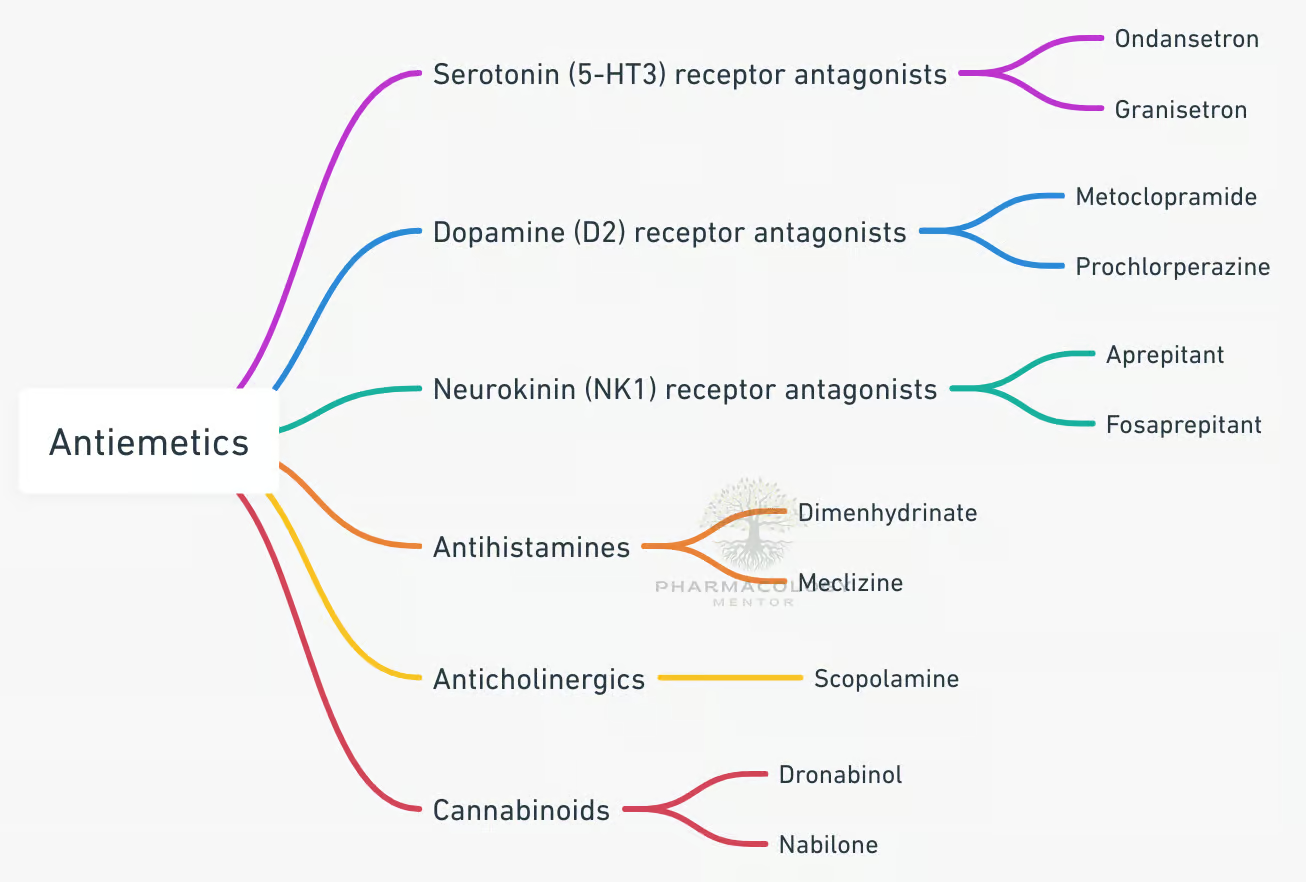
Classification of Antiemetic Drugs
1. Serotonin (5-HT₃) Receptor Antagonists
Examples: Ondansetron, Granisetron, Palonosetron.
These agents selectively block 5-HT₃ receptors in both peripheral vagal nerve terminals and centrally in the CTZ, curtailing acute CINV and postoperative nausea and vomiting (PONV). They revolutionized oncology care by substantially mitigating emetic reflexes triggered by chemotherapeutic regimens. Palonosetron, featuring an extended half-life (~40 hours) and higher receptor affinity, shows sustained effect for delayed-phase CINV, particularly in synergy with other antiemetics.
2. Dopamine (D₂) Receptor Antagonists
This class comprises multiple subgroups:
- Phenothiazines (e.g., Prochlorperazine, Chlorpromazine): Broad-spectrum antiemetics that block D₂ receptors in the CTZ, with additional anticholinergic and antihistaminic properties.
- Butyrophenones (e.g., Droperidol, Haloperidol): Potent D₂ blockade in the CTZ; droperidol is widely recognized for controlling PONV, though concerns about QT prolongation demand caution.
- Benzamides (e.g., Metoclopramide, Domperidone): Metoclopramide exerts central D₂ blockade and peripheral prokinetic activity by sensitizing GI tissues to acetylcholine. Domperidone, less lipophilic, has fewer central side effects but still antagonizes D₂ receptors in the periphery and area postrema.
3. Neurokinin-1 (NK₁) Receptor Antagonists
Examples: Aprepitant, Fosaprepitant, Rolapitant.
They prevent substance P from binding to NK₁ receptors in the CTZ and vomiting center, reducing both acute and delayed CINV. Commonly used in combination with 5-HT₃ antagonists and corticosteroids for highly emetogenic chemotherapy (e.g., cisplatin).
4. Histamine (H1) and Muscarinic (M1) Antagonists
Examples: Dimenhydrinate, Meclizine, Scopolamine.
These are classical choices for motion sickness by blocking M1 and H1 receptors in the vestibular system. While effective, they often cause sedation, dry mouth, blurred vision, or other anticholinergic side effects.
5. Corticosteroids
Dexamethasone is frequently used, especially in chemotherapy regimens. Although the precise mechanism is not fully elucidated, decreased prostaglandin and serotonin release, stabilized blood-brain barrier, and immunosuppressive effects likely contribute.
6. Benzodiazepines
Lorazepam, Alprazolam, used as adjuncts for anticipatory nausea or anxiety-related vomiting. They reduce the psychological components of emesis but possess limited direct antiemetic properties.
7. Cannabinoids
Examples: Dronabinol, Nabilone.
These synthetic delta-9-tetrahydrocannabinol (THC) analogs act on cannabinoid (CB1) receptors in the CNS. They are particularly helpful for refractory CINV, although sedation, euphoria, and dysphoria might limit their routine use.
8. Other or Adjunctive Agents
Agents such as Olanzapine (an atypical antipsychotic) block multiple receptor subtypes (dopamine, serotonin, histamine), proving beneficial in highly emetogenic chemotherapy or in multi-drug regimens that have failed standard therapies.
Mechanisms of Action in Detail
5-HT₃ Receptor Antagonists
When the GI tract is insulted by chemotherapy or radiation, serotonin is released from enterochromaffin cells, activating 5-HT₃ receptors on vagal afferents. By blocking these receptors in the gut and in the CTZ, drugs like Ondansetron and Granisetron halt peripheral and central emetic signals (Goodman & Gilman’s, 13th Edition). Palonosetron exerts particularly prolonged receptor binding, enhancing efficacy against delayed emesis.
Dopamine Antagonists
Prochlorperazine and Droperidol mitigate dopamine-mediated triggers in the CTZ; meanwhile, Metoclopramide also boosts upper GI tone and accelerates gastric emptying (Katzung, 15th Edition). However, their blockade of D₂ in the nigrostriatal pathway can precipitate extrapyramidal effects (EPS), including acute dystonia or tardive dyskinesia with long-term usage.
Neurokinin-1 (NK₁) Antagonists
While serotonin is pivotal to the acute phase of CINV, the delayed phase is significantly driven by substance P. Aprepitant (oral) or fosaprepitant (IV) selectively blocks NK₁ receptors in the brainstem (Rang & Dale’s, 8th Edition), diminishing sustained emesis. NK₁ antagonists work synergistically with 5-HT₃ antagonists and dexamethasone, significantly raising antiemetic control in high-risk chemotherapy protocols.
Histamine and Muscarinic Antagonists
Motion sickness arises from mismatched sensory signals in the vestibular apparatus; H1 and M1 blockade with meclizine, dimenhydrinate, or scopolamine attenuates labyrinthine-mediated stimulation of the vomiting center. Scopolamine’s transdermal delivery can ensure stable prophylaxis for extended travel scenarios, though dryness of mouth, sedation, and blurred vision are notable side effects.
Corticosteroids
Dexamethasone confers antiemetic synergy with 5-HT₃-blockers—particularly beneficial for prophylaxis in moderate-to-high emetic risk regimens. Mechanisms include decreased inflammatory mediator release and stabilized BBB function (Goodman & Gilman’s, 13th Edition). The short-term usage typical in antiemetic protocols often spares patients from steroid’s more chronic adverse effects.
Benzodiazepines
Anxiolysis, sedation, and anterograde amnesia help Lorazepam or Alprazolam reduce anticipatory vomiting in patients who have experienced repeated emetic episodes with prior treatments (Katzung, 15th Edition). Their utility is especially high in the psychological context of chemotherapy.
Cannabinoids
Dronabinol activates central cannabinoid CB1 receptors, suppressing the vomiting reflex. Appetite stimulation is a side “benefit” for some patients, while sedation, euphoria, and potential psychosis hamper broader acceptance. Typically reserved for refractory cases or when conventional agents fail.
Pharmacokinetics of Key Agents
Ondansetron
- Absorption: High oral bioavailability; also IV/IM forms.
- Metabolism: Hepatic, via CYP3A4, 1A2, 2D6.
- Half-Life: ~3–5 hours.
- Administration: Typically pre-chemotherapy or preoperatively, plus repeated doses if needed for protracted risk.
Metoclopramide
- Absorption: Rapid from GI for oral dosing; also given IV/IM.
- Metabolism: Hepatic.
- Half-Life: ~5–6 hours.
- Clinical Note: Risk of extrapyramidal side effects with prolonged or high doses; also used for gastroparesis management.
Aprepitant
- Absorption: Good oral bioavailability.
- Metabolism: Predominantly by CYP3A4, raising concerns about drug interactions (e.g., with warfarin, certain chemotherapeutics).
- Half-Life: ~9–13 hours.
- Administration: Generally begun prior to chemotherapy and continued for a few days to cover delayed emesis.
Dimenhydrinate (H1 Antagonist)
- Absorption: Orally well absorbed; also available in rectal form.
- Onset: ~30 min to 1 hour.
- Duration: Typically 4–6 hours.
- Side Effects: Drowsiness, sedation, anticholinergic phenomena.
Scopolamine
- Administration: Transdermal typically, providing sustained release.
- Systemic Levels: Gradual absorption avoiding peaks that might cause excessive sedation.
- Duration: Up to 72 hours per patch.
Clinical Applications
1. Chemotherapy-Induced Nausea and Vomiting (CINV)
CINV is divided into acute (<24 hours) and delayed (24–120 hours) phases. For highly emetogenic chemotherapy (e.g., cisplatin-based), triple regimens combining an NK₁ antagonist (Aprepitant), a 5-HT₃ antagonist (Ondansetron or Palonosetron), and dexamethasone yield robust prophylaxis (Katzung, 15th Edition). Lower-risk chemotherapy may need fewer agents (e.g., single 5-HT₃ antagonist + dexamethasone). Olanzapine may also be used as an alternative or adjunct in refractory or highly emetogenic conditions.
2. Postoperative Nausea and Vomiting (PONV)
Up to a third of surgical patients can experience PONV, rising to 70–80% with multiple risk factors (female, nonsmoker, prior PONV, use of volatile anesthetics/opioids). 5-HT₃ antagonists (e.g., Ondansetron), dexamethasone, and low-dose droperidol or haloperidol are common prophylactic measures. For rescue, additional classes (NK₁ antagonist, antihistamine) might be appropriate if first-line fails.
3. Radiation-Induced Emesis
Radiation to the abdomen or total-body radiation can induce emesis similarly to chemotherapy. 5-HT₃ antagonists plus or minus steroids are the backbone for prophylaxis and treatment. Palonosetron’s prolonged effect can be advantageous for fractionated radiotherapy regimens (Rang & Dale’s, 8th Edition).
4. Motion Sickness and Vestibular Disorders
Scopolamine patches placed behind the ear and H1-blockers (Meclizine, Dimenhydrinate) effectively curb labyrinthine overstimulation. They must be taken prophylactically, ideally before traveling or exposure to motion, as reversing established motion sickness is more challenging.
5. Pregnancy-Related Nausea and Vomiting
For mild to moderate morning sickness, Vitamin B6 (pyridoxine), doxylamine, or ginger can suffice. In hyperemesis gravidarum, or more severe forms, short courses of ondansetron, metoclopramide, or promethazine might be employed. A balanced risk-benefit approach is mandatory due to fetal safety concerns (Goodman & Gilman’s, 13th Edition).
6. Gastroenteritis and Other Causes
Acute gastroenteritis may benefit from selective 5-HT₃ blockade (e.g., Ondansetron) to mitigate severe vomiting, particularly in pediatric dehydration. Meanwhile, prokinetics like metoclopramide help in functional dyspepsia and gastroparesis, alleviating associated nausea.
7. Anticipatory Nausea
Repeated cycles of chemotherapy can lead to anticipatory nausea triggered by environmental cues. Benzodiazepines (e.g., Lorazepam) reduce anxiety, sedation can block conditioned responses. Relaxation techniques or mental imagery also assist in controlling this phenomenon.
Adverse Effects and Precautions
Dopamine Antagonists (Phenothiazines, Butyrophenones, Benzamides)
- Sedation, orthostatic hypotension, significant extrapyramidal symptoms (EPS) including acute dystonia, akathisia, and with chronic use, tardive dyskinesia.
- Low-dose usage or prophylactic antihistamines (like diphenhydramine) may lower EPS incidence.
5-HT₃ Antagonists
- Headache, constipation, mild sedation.
- QT prolongation potential with some agents. Caution in patients with congenital Long QT syndrome or concurrent QT-prolonging medications.
NK₁ Antagonists
- Common side effects: Fatigue, hiccups, dyspepsia.
- Notable interactions due to CYP3A4 metabolism. Monitoring is crucial if coadministered with warfarin or certain chemotherapeutics.
Anticholinergics (M1) and Antihistamines (H1)
- Sedation, dry mouth, blurred vision, urinary retention.
- In older adults, risk of confusion or exacerbation of narrow-angle glaucoma or prostatic hyperplasia.
Corticosteroids
- Insomnia, mood changes, hyperglycemia. Usually well-tolerated short-term. Prolonged usage could lead to immunosuppression, Cushingoid features, or osteoporosis.
Benzodiazepines
- Drowsiness, sedation, risk of dependence or withdrawal symptoms if used long-term.
- Can cause respiratory depression if combined with opioids or in compromised patients.
Cannabinoids
- Euphoria, sedation, cognitive impairment, potential for dysphoria or psychotomimetic effects in some individuals.
Rational Combination Therapy
For highly emetogenic chemotherapy, a three-drug regimen including an NK₁ antagonist, a 5-HT₃ antagonist, and dexamethasone is standard. This synergy addresses multiple pathways:
- Immediate release of serotonin from enterochromaffin cells (blocked by 5-HT₃ antagonists).
- Substance P-driven delayed emesis (inhibited by NK₁ antagonists).
- General anti-inflammatory and possible downstream antiemetic actions (enhanced by steroids).
Additional or rescue antiemetics (e.g., metoclopramide, olanzapine) address breakthrough or refractory vomiting. For moderate-risk chemotherapy, dual regimens (5-HT₃ antagonist + dexamethasone) might suffice. Adapting prophylaxis to emetic risk categories and patient-specific risk factors is key.
Special Populations
Pediatrics
Ondansetron is widely utilized for pediatric gastroenteritis or chemotherapy. Weight-based dosing helps mitigate dosing errors. Watch for fluid deficits or electrolyte imbalances masked by reduced vomiting. The sedation threshold can differ from adults, so carefully monitor for sedation or paradoxical excitement with antihistamines.
Geriatrics
Greater vulnerability to sedation, confusion, or anticholinergic side effects. For instance, scopolamine or chlorpromazine might provoke delirium in cognitively fragile patients. Dose adjustments or alternative agents with fewer CNS or cardiovascular effects are prudent.
Pregnancy and Lactation
Vitamin B6 (pyridoxine) and doxylamine are first-line for morning sickness. For severe hyperemesis gravidarum, ondansetron or promethazine may be used after evaluating fetal safety. Metoclopramide is also employed. Minimizing anticholinergic or sedation burden is essential for maternal well-being.
Hepatic/Renal Impairment
Many antiemetics (e.g., ondansetron, aprepitant) undergo hepatic metabolism, necessitating caution or dose modifications. Metoclopramide clearance is also impacted by renal function.
Evolving Frontiers and Future Directions
Novel Receptor Targets
Ongoing research explores potential roles of ghrelin antagonists, endocannabinoid modulators, or refined substance P inhibitors for more robust antiemetic coverage. Investigational compounds aim to address refractory N/V or emergent therapies with yet-unmet needs.
Pharmacogenomics
Individual genetic differences in drug metabolism (e.g., CYP2D6, CYP3A4 polymorphisms) or receptor polymorphisms might soon facilitate personalized antiemetic strategies, optimizing drug selection, dosage, and combination for each patient.
Extended-Release Formulations and Alternate Delivery
Transdermal or subcutaneous on-body injections of granisetron, sublingual or intranasal formulations of other antiemetics, and next-generation NK₁ antagonists may offer more convenient prophylaxis, especially in outpatient or palliative care contexts.
Combination with Non-Pharmacological Methods
Relaxation training, cognitive-behavioral therapy, acupuncture, or ginger supplementation can augment pharmaceutical measures. Integrated care strategies aim to minimize polypharmacy while preserving improved patient outcomes and quality of life (Goodman & Gilman’s, 13th Edition).
Practical Clinical Tips
- Identify Emetic Risk: For oncology or surgical contexts, stratify risk of emesis (none, low, moderate, high) to guide prophylaxis intensity.
- Prophylaxis vs. Rescue: Administer prophylactic antiemetics proactively for high-risk procedures or chemotherapies. Once vomiting is established, controlling it becomes more challenging.
- Combine Classes: Tackle multiple pathways—Serotonin, NK₁, Dopamine—to achieve synergy and reduce reliance on higher doses of a single agent.
- Monitor QT Interval: Some 5-HT₃ antagonists or droperidol may prolong QT, necessitating caution in susceptible individuals.
- Watch for EPS: If prescribing D₂ antagonists, especially in younger or older patients, be vigilant for early extrapyramidal reactions (dystonic reactions, akathisia).
- Assess Drug Interactions: NK₁ antagonists heavily utilize CYP3A4. Co-administration with certain oncologic or anti-infective agents can affect plasma levels.
- Adapt for Comorbidities: Geriatrics require lower doses to avoid sedation or confusion. Patients with narrow-angle glaucoma or urinary retention may fare poorly with anticholinergics.
- Patient Education: Emphasize correct usage timelines (e.g., scopolamine patch application before travel), potential sedation, and the need to avoid tasks requiring alertness if sedation is expected.
Conclusion
Antiemetic pharmacology forms a vital segment of symptom control in medicine, shaping treatment tolerance and patient well-being in oncology, perioperative practice, motion sickness, GI infections, and beyond. With expanding insights into the complex neurochemical interplay underlying nausea and vomiting, we now possess a formidable arsenal of agents—from 5-HT₃ antagonists and NK₁ antagonists to dopamine D₂ blockers, H1/M1 antagonists, corticosteroids, and cannabinoids. Rational combination therapy—tailored to emetic risk profiles, comorbidities, and drug metabolism—can achieve impressive success in preventing or relieving emesis.
Potential pitfalls include sedation, extrapyramidal symptoms, anticholinergic burdens, QT prolongation, and clinically relevant drug interactions. Nonetheless, advanced formulations, improved receptor selectivity, and synergy with integrative measures continue to refine antiemetic practice. By comprehensively applying the knowledge from authoritative texts (Goodman & Gilman’s, Katzung, Rang & Dale) and emerging research, clinicians are better equipped to implement potent, individualized antiemetic care, thus safeguarding patient comfort, nutritional status, and overall therapeutic success.
Book Citations
Rang HP, Dale MM, Rang & Dale’s Pharmacology (8th Edition).
Goodman & Gilman’s The Pharmacological Basis of Therapeutics (13th Edition).
Katzung BG, Basic & Clinical Pharmacology (15th Edition).