Introduction
Testosterone is a key steroid hormone primarily secreted by the testes in males and, to a lesser extent, by the ovaries in females and the adrenal glands in both sexes. It is the most important androgen, critically influencing the development and maintenance of male secondary sexual characteristics, muscle mass, bone density, and overall metabolic health. Beyond reproductive and sexual function, testosterone also regulates mood, cognitive function, and body composition, underscoring its broad physiological relevance.
Clinically, physicians have leveraged testosterone replacement therapy (TRT) and other androgenic drugs to address conditions such as hypogonadism, delayed puberty, and certain rare cases of catabolic states. However, the robust anabolic properties of testosterone also open the door to misuse, particularly in sports doping. Moreover, decisions around hormone therapy must carefully balance benefits (enhanced libido, muscle strength, well-being) with potential adverse effects (prostate disorders, cardiovascular risks, or hepatic strain). The pharmacology of testosterone is multifaceted, encompassing its biosynthesis, pathways of action, metabolic processes, clinical indications, formulation challenges, and ramifications of long-term use.
This comprehensive overview will explore the pharmacology of testosterone: from its natural production and mechanism of action to pharmacokinetics, therapeutic uses, side effect profiles, and future research directions.
Historical Context and Background
The study of testosterone began in earnest during the late 19th and early 20th centuries, when scientists observed its role in male sexual differentiation and male characteristic development. However, it was not until 1935 that testosterone was first isolated, synthesized, and identified as the principal male sex hormone. Subsequently, the recognition of its anabolic and androgenic activities spurred the development of synthetic derivatives for clinical use.
By the mid-20th century, testosterone was used experimentally in conditions like hypogonadism and certain debilitating illnesses to preserve muscle mass and improve energy. Over time, controversies emerged due to anabolic steroid abuse in sports. Yet, legitimate medical applications of testosterone have continued to expand, supported by improved delivery methods (transdermal patches, topical gels, long-acting injections) and more nuanced understanding of androgen physiology.
Structure and Biosynthesis
Steroid Structure
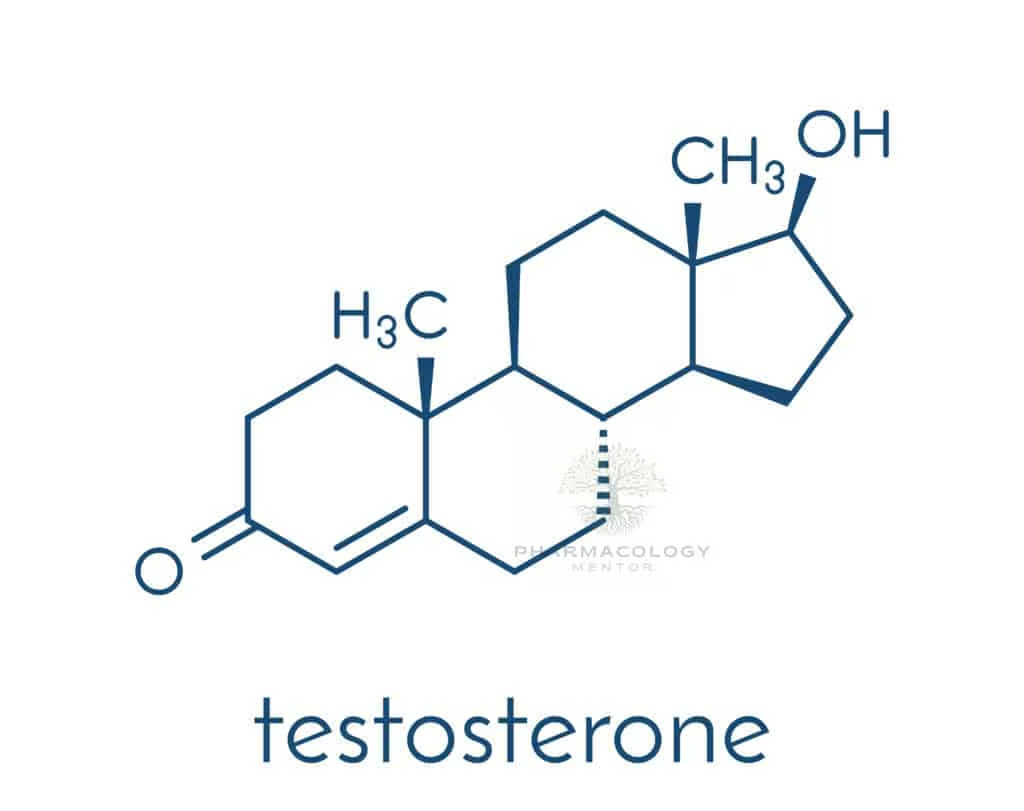
Testosterone belongs to the class of C19 steroids. It has a four-ring structure typical of all steroids, with characteristic modifications that confer androgenic properties. The molecular structure shows a keto group at carbon 3 and a double bond between carbons 4 and 5, distinguishing it from other steroid families (e.g., estrogens, progestins).
Biosynthetic Pathway
- Cholesterol is the precursor for all steroid hormones. Within Leydig cells of the testes, cholesterol is transported into mitochondria, where it is converted to pregnenolone by the enzyme cholesterol desmolase.
- Pregnenolone is further transformed through several enzymatic steps (involving 3β-hydroxysteroid dehydrogenase, 17α-hydroxylase) into androstenedione.
- The enzyme 17β-hydroxysteroid dehydrogenase (17β-HSD) then reduces androstenedione to testosterone.
In men, approximately 95% of testosterone is synthesized in the testes, with the remainder derived from the adrenal cortex. In women, the primary sources include the ovaries and adrenal glands. Despite lower circulating levels in females, testosterone remains an important hormone for libido, bone health, and muscle function.
Regulation
The hypothalamic-pituitary-gonadal (HPG) axis modulates testosterone production. Gonadotropin-releasing hormone (GnRH) from the hypothalamus stimulates the anterior pituitary to secrete luteinizing hormone (LH), which in turn acts on Leydig cells to increase testosterone synthesis. Negative feedback arises from elevated testosterone levels (and its aromatized product, estradiol) on both the pituitary and hypothalamus, providing tight physiologic control.
Mechanism of Action
Testosterone exerts its effects through androgen receptors (AR)—nuclear transcription factors found in various tissues, including muscle, bone, prostate, and central nervous system. Two main mechanisms of androgenic signaling prevail:
- Direct Binding to the Androgen Receptor
Testosterone diffuses into the cytoplasm of target cells, where a portion directly binds the androgen receptor. After receptor binding, the complex undergoes conformational change, dissociates from heat shock proteins, dimerizes, and translocates to the nucleus. There, it binds specific androgen response elements (AREs) on DNA, modulating gene transcription and protein synthesis. - Conversion to More Potent Androgens or Estrogens
- Dihydrotestosterone (DHT): In some tissues (e.g., prostate, skin), the enzyme 5α-reductase transforms testosterone to DHT, which binds the androgen receptor with higher affinity. This pathway is key for prostate growth, male external genitalia development, and certain aspects of male pattern hair.
- Estradiol: Through aromatase action, testosterone can convert to 17β-estradiol, which exerts feedback on the HPG axis and influences bone mass, libido, and other processes traditionally associated with estrogen.
Differences in local enzyme expression (e.g., 5α-reductase, aromatase) underlie tissue-specific actions of testosterone. Thus, testosterone can be viewed as a prohormone for DHT and estradiol in addition to being an active androgen in its own right.
Pharmacokinetics of Testosterone
Absorption
Testosterone is well absorbed through the gastrointestinal tract but undergoes substantial first-pass metabolism in the liver, leading to poor oral bioavailability. Early attempts at oral formulations were mostly unsuccessful unless the molecule was chemically modified (e.g., 17-alkylated derivatives) to resist hepatic breakdown. However, such modifications can carry a risk of hepatotoxicity.
Modern approaches favor parenteral or transdermal routes to circumvent first-pass metabolism. Common methods include:
- Intramuscular (IM) injections of testosterone enanthate or testosterone cypionate
- Transdermal patches
- Topical gels
- Buccal tablets
- Subcutaneous pellets (long-acting)
Distribution
Once in circulation, testosterone is bound approximately 98% to plasma proteins—principally sex hormone-binding globulin (SHBG) and albumin. Only the free fraction (~2%) or the albumin-bound fraction (loosely bound, thus biologically accessible) exerts physiological effects.
Metabolism
Testosterone undergoes metabolism primarily in the liver. Key pathways include:
- Reduction to androsterone and etiocholanolone, excreted in the urine as conjugates.
- 5α-reduction to DHT in specific tissues.
- Aromatization to estradiol.
Hepatic clearance is high, explaining the difficulty in maintaining stable blood levels after oral dosing. Titration strategies for parenteral routes aim to achieve physiologic or near-physiologic serum concentrations.
Excretion
Metabolites are largely renally excreted, with a smaller fraction eliminated via bile/feces. In individuals with advanced liver disease or renal dysfunction, drug clearance can be altered, influencing testosterone therapy dosing and side effect risk.
Physiological and Clinical Effects

Androgenic Actions
- Development of Male External Genitalia: Fetal differentiation in utero.
- Pubertal Changes: Growth of testes, penis, scrotum; deepening of the voice; facial/pubic hair.
- Maintenance of Secondary Sexual Characteristics: Beard growth, male pattern hair distribution.
- Stimulation of Sebaceous Glands: Possibly leading to acne in adolescents or adult onset.
- Prostate Health: Growth and maintenance; can exacerbate benign prostatic hyperplasia (BPH) or prostate cancer.
Anabolic Actions
Testosterone augments protein synthesis, thereby enhancing:
- Muscle Mass and Strength
- Red Blood Cell Production (erythropoiesis)
- Bone Density (reducing osteoporotic risk)
These effects explain why testosterone or its derivatives can be employed clinically in wasting conditions but also highlight its abuse as an anabolic steroid by some athletes.
Metabolic Functions
Testosterone influences fat distribution, glucose metabolism, and lipid profiles. Low testosterone correlates with increased visceral adiposity, insulin resistance, and dyslipidemia in many men, spurring interest in testosterone replacement to improve metabolic health—although robust evidence remains mixed.
CNS and Neurobehavioral Effects
Androgens modulate libido, mood, and certain cognitive functions. Severe androgen deficiency can be accompanied by depressive symptoms, reduced sexual desire, or fatigue. Restoration of normal levels can rectify such deficits, but the relationship between testosterone and behavior (especially aggression) is complex and influenced by numerous psychosocial factors.
Clinical Indications for Testosterone Therapy
- Male Hypogonadism
- Primary Hypogonadism (testicular failure): Elevated LH/FSH with low testosterone. Examples include Klinefelter syndrome, testicular injury, or orchiectomy.
- Secondary Hypogonadism (pituitary or hypothalamic dysfunction): Low or inappropriately normal LH/FSH leads to insufficient testosterone production.
- Delayed Puberty
Testosterone therapy can induce or accelerate pubertal changes in adolescent boys who have delayed onset, whether due to constitutional growth delay or hypogonadism. Low-dose regimens and careful monitoring to ensure harmonious growth are crucial. - HIV/AIDS Wasting or Other Catabolic States
Some clinicians employ testosterone or other anabolic agents to counteract the catabolism and muscle loss that accompany chronic illnesses such as advanced HIV infection. - Transgender Hormone Therapy
Female-to-male (FTM) individuals may receive testosterone to induce virilization, including facial hair growth, voice deepening, and body fat redistribution. Dosing strategies are guided by desire for masculine secondary sexual characteristics and monitoring for side effects. - Male Contraception Research
Investigational regimens combining testosterone with progestins can produce gonadotropin suppression, potentially offering a reversible male contraceptive approach. While promising, these have not gained widespread regulatory approval.
Off-Label or Controversial Uses
- Aging Males with Low Testosterone: Clinics offering “testosterone for vitality” have proliferated, but robust evidence on long-term benefits versus risks (e.g., cardiovascular events, prostate health) remains inconclusive.
- Sexual Dysfunction: For men with hypogonadism-related erectile dysfunction or low libido, testosterone can help. However, in eugonadal men with ED, PDE5 inhibitors (e.g., sildenafil) are generally first-line.
- Athletic Performance Enhancement: Strictly prohibited by sports governing bodies due to doping issues and substantial health risks.
Preparations and Administration Routes
Injectable Esters
- Testosterone Enanthate and Testosterone Cypionate: Administered intramuscularly every 2–4 weeks. Provide a dose-related surge in levels post-injection, followed by a tail-off.
- Testosterone Undecanoate: A long-acting IM formulation, achieving more stable levels over ~10–14 weeks. Requires caution regarding injection-related pulmonary oil microembolism.
Transdermal Applications
- Patches (e.g., Androderm): Applied daily, produce relatively stable levels, though local skin irritation can occur.
- Gels (e.g., AndroGel, Testim): Popular due to ease of use and stable serum testosterone. However, risk of transference to others via direct contact demands caution.
Oral or Buccal Preparations
- Modified Oral Testosterone (Undecanoate): Absorbed via lymphatic system, bypassing part of first-pass metabolism. Still subject to variable absorption.
- Buccal Tablets: Attach to gum and release hormone slowly. Less commonly used.
Implants
- Pellets (Testopel): Subcutaneous insertion in the hip region. Provide sustained release over 3–6 months. A minimally invasive procedure but can cause local discomfort or pellet extrusion.
Criteria for Choosing a Formulation
Factors include patient preference, cost, convenience, the desired pharmacokinetic profile, and minimization of side effects (e.g., peaks/troughs with shorter-acting injectables). Proper patient education is essential to assure compliance and safety.
Adverse Effects of Testosterone Therapy
Common Side Effects
- Acne and oily skin (enhanced sebaceous gland activity)
- Fluid Retention or mild edema
- Erythrocytosis: Elevated hematocrit, potentially increasing thrombotic risk if hematocrit rises above 54–55%
- Mood Alterations: Some individuals report changes in aggression or irritability
Prostate Concerns
Chronic androgen exposure may accelerate benign prostatic hyperplasia (BPH) progression or unmask previously undiagnosed prostate cancer. Regular digital rectal exams (DRE) and PSA (prostate-specific antigen) monitoring are recommended in older males on long-term TRT.
Cardiovascular Risks
Debate persists about a potential link between testosterone therapy and cardiovascular events (myocardial infarction, stroke). Some observational studies suggest increased risk in older men with pre-existing heart disease; others find no significant risk or even marginal benefit. Ongoing research and cautious patient selection remain critical.
Liver Toxicity
While native testosterone is not highly hepatotoxic, 17α-alkylated androgens (e.g., methyltestosterone, oxymetholone) can cause cholestasis, peliosis hepatis, and hepatic neoplasms with prolonged misuse. Such formulations are generally avoided in modern clinical practice.
HPG Axis Suppression and Infertility
Exogenous testosterone suppresses gonadotropin release, leading to decreased intratesticular androgens, lowered sperm production, and testicular atrophy. In men desiring fertility, co-treatment with gonadotropins or the use of selective modulators (e.g., clomiphene) may be indicated instead of purely exogenous androgens.
Psychosocial Adverse Effects
Cases of testosterone-induced mania or aggressive behavior in predisposed individuals have been reported, particularly at supraphysiologic dosages. Monitoring mental health is important in certain populations prone to mood dysregulation.
Contraindications and Precautions
- Prostate Cancer: Androgens can stimulate tumor growth. Confirming the absence of prostate malignancy is strongly advised.
- Severe BPH or Lower Urinary Tract Symptoms**: Worsening obstruction can occur.
- Polycythemia or Hematocrit > 50%**: Heightened risk of hyperviscosity-related events.
- Uncontrolled Heart Failure: Fluid retention might exacerbate symptoms.
- Pregnancy/Breastfeeding: Androgens are contraindicated due to masculinization of the fetus and other unknown risks.
- Breast Cancer in Males: Rare but test is generally contraindicated unless specifically indicated.
Risk-to-benefit assessments should be performed methodically, integrating baseline labs (lipids, liver function, complete blood count, PSA) and ongoing monitoring.
Testosterone Abuse and Anabolic Steroid Misuse
Testosterone and its synthetic analogs are abused among some athletes and bodybuilders for performance enhancement. Doses far exceed therapeutic ranges, potentially culminating in:
- Liver Damage (with 17α-alkylated agents)
- Severe Acne
- Psychiatric Issues (“Roid rage,” mood swings)
- Cardiomyopathy and arterial hypertension
- Dyslipidemia (reduced HDL, increased LDL)
- Testicular Atrophy and Infertility
International sports organizations have banned anabolic steroids, employing doping tests analyzing T/E (testosterone/epitestosterone) ratios, carbon isotope ratio mass spectrometry, and other methods to detect exogenous testosterone administration.
Monitoring and Follow-Up in Testosterone Therapy
Clinicians should adopt a structured follow-up schedule to monitor:
- Serum Testosterone Levels: Assess trough values for injectables or check steady-state for gels/patches. Aim for mid-physiologic range (roughly 400–700 ng/dL in adult males).
- Hematocrit: Discontinue or reduce dose if hematocrit exceeds 52–54%.
- PSA and Prostate Assessment: DRE and PSA measurement are indicated at baseline, then at 3–6 months and annually if stable.
- Cardiometabolic Profile: Check lipids, glycemic control, blood pressure.
- Symptom Improvement: Evaluate sexual function, mood, muscle strength, bone density (if indicated).
Compliance and follow-through are essential for safe, effective therapy. Hormone regimens must be individualized, with ongoing discussion about risks, benefits, and patient concerns.
Future Directions and Research
Novel Formulations
Researchers continue to explore improved testosterone delivery systems with stable pharmacokinetics, minimal injection frequency, and fewer side effects. Microencapsulation, selective androgen receptor modulators (SARMs), and advanced transdermal technologies represent future frontiers.
Selective Androgen Receptor Modulators (SARMs)
SARMs aim to segregate anabolic from androgenic effects, conferring muscle/bone benefits without robust androgenic impact on the prostate or hair pattern. While promising, widespread clinical adoption of SARMs faces regulatory challenges and long-term safety questions.
Androgens in Women’s Health
Low-dose testosterone therapy for hypoactive sexual desire disorder (HSDD) in women remains controversial. Better delineation of dosing, efficacy, and safety in females requires further rigorously designed studies.
Genetic and Pharmacogenomic Insights
Differential sensitivity to testosterone among individuals highlighted by androgen receptor polymorphisms (e.g., CAG repeat length) might eventually enable personalized endocrine therapies. Pharmacogenomic profiling could guide dose adjustments and predict those at higher risk for adverse events.
Conclusion
Testosterone is integral to male physiology, catalyzing puberty, maintaining sexual function, and building musculoskeletal integrity. Its spheres of influence also extend to mood, cognition, and metabolic homeostasis. Clinical use of testosterone or androgenic analogs significantly improves outcomes in men with documented hypogonadism and provides essential hormone support in gender-affirming care. Yet, the decision to initiate therapy, formulation selection, and dosing involve balancing recognized benefits (enhanced libido, improved bone density/muscle mass, alleviation of hypogonadal symptoms) against potential setbacks (fluid retention, erythrocytosis, prostate complications, potential cardiovascular uncertainties).
Contemporary practice capitalizes on diverse administration routes—from long-acting injectables to transdermal gels—enabling a more individualized approach. Monitoring guidelines emphasize PSA, hematocrit, and robust clinical assessments, safeguarding patient well-being while ensuring effective symptom relief. Meanwhile, misuse of testosterone remains a considerable public health concern, underscoring the need for education and stricter regulation.
Looking forward, the pursuit of SARMs and advanced formulations could refine the art of androgen therapy. More robust data on broader populations—women, older males, and those with co-morbidities—will fill gaps in understanding and yield refined clinical guidelines. As our knowledge of testosterone’s physiologic and pathophysiologic roles grows, practitioners can deploy therapy with greater precision, harnessing the hormone’s essential contributions to human health while sharply reducing associated risks.
Book Citation
Katzung BG, Basic & Clinical Pharmacology, 15th Edition.









