Introduction
Testosterone is a key hormone in the human body, primarily recognized for its role in male sexual development and secondary sexual characteristics. However, its pharmacological profile extends well beyond basic reproduction and development. Testosterone exerts significant influence on muscle mass, bone density, red blood cell production, and even certain aspects of behavior. Understanding its pharmacology is pivotal not only for the management of conditions like hypogonadism but also for broader hormone replacement therapies and research into aging processes.
In modern clinical practice, testosterone preparations are used across a spectrum of applications, from treating delayed puberty to mitigating the impact of chronic conditions that reduce testosterone levels. With increasing interest in hormone replacement therapy (HRT) and the growing population of older adults, optimizing testosterone therapy while minimizing side effects has become a primary goal in endocrinology and pharmacology.
This article provides a comprehensive exploration of testosterone’s pharmacology at a depth suitable for students, clinicians, and researchers. By the end, you will have gained a thorough understanding of how testosterone works, how it is metabolized, and why it is central to various therapeutic strategies.
Historical Perspective
The pharmacology of testosterone can be traced back to ancient times, even if our ancestors did not fully comprehend its functions. Early civilizations recognized that the testes were vital for masculinity and virility. Scientific inquiry into the hormone’s physiological significance began in the 19th century, but it was not until the early 20th century that researchers identified testosterone as a discrete biochemical entity.By the 1930s, testosterone had been chemically synthesized, setting the stage for groundbreaking clinical applications. Early clinical experiments showed that testosterone had both anabolic (muscle-building) and androgenic (masculinizing) properties. Over the decades, refinements in pharmaceutical formulations have allowed the development of multiple delivery mechanisms—from oral tablets to transdermal patches to injectable esters—each with distinct pharmacokinetic profiles. These historical milestones reflect the hormome’s complex interplay within the endocrine system (Katzung & Trevor, “Basic and Clinical Pharmacology,” 14th ed.).
Endogenous Production of Testosterone
Biosynthesis
Testosterone biosynthesis primarily occurs in the Leydig cells of the testes under the influence of luteinizing hormone (LH) secreted by the anterior pituitary gland. Cholesterol is the starting substrate for testosterone production, which requires a variety of enzymes, including P450scc (cholesterol side-chain cleavage enzyme) and 17β-hydroxysteroid dehydrogenase.
Regulation
The hypothalamic-pituitary-gonadal (HPG) axis tightly regulates testosterone levels:
- The hypothalamus releases gonadotropin-releasing hormone (GnRH).
- GnRH stimulates the pituitary to secrete LH and follicle-stimulating hormone (FSH).
- LH stimulates Leydig cells to produce testosterone.
- Testosterone exerts negative feedback on both the hypothalamus and pituitary to maintain homeostasis (Guyton & Hall, “Textbook of Medical Physiology,” 13th ed.).
This feedback loop ensures consistent testosterone levels within a physiologic range. Abnormalities in this axis, such as pituitary adenomas or testicular damage, can decrease testosterone production, leading to disorders like hypogonadism.
Chemistry and Structure
Testosterone (17β-hydroxy-4-androsten-3-one) belongs to the androgen class of hormones and is structurally derived from cholesterol. It has a four-ring carbon skeleton common to all steroids, comprising three six-member rings (A, B, and C) and one five-member ring (D). Small structural modifications to the basic steroid framework can produce dramatic changes in hormone function. For instance, estradiol, an estrogen, differs from testosterone only by a single methyl group and the presence of an aromatic ring.
Key structural points include:
- The presence of a keto group at carbon 3.
- A hydroxyl group at carbon 17.
- A double bond between carbons 4 and 5.
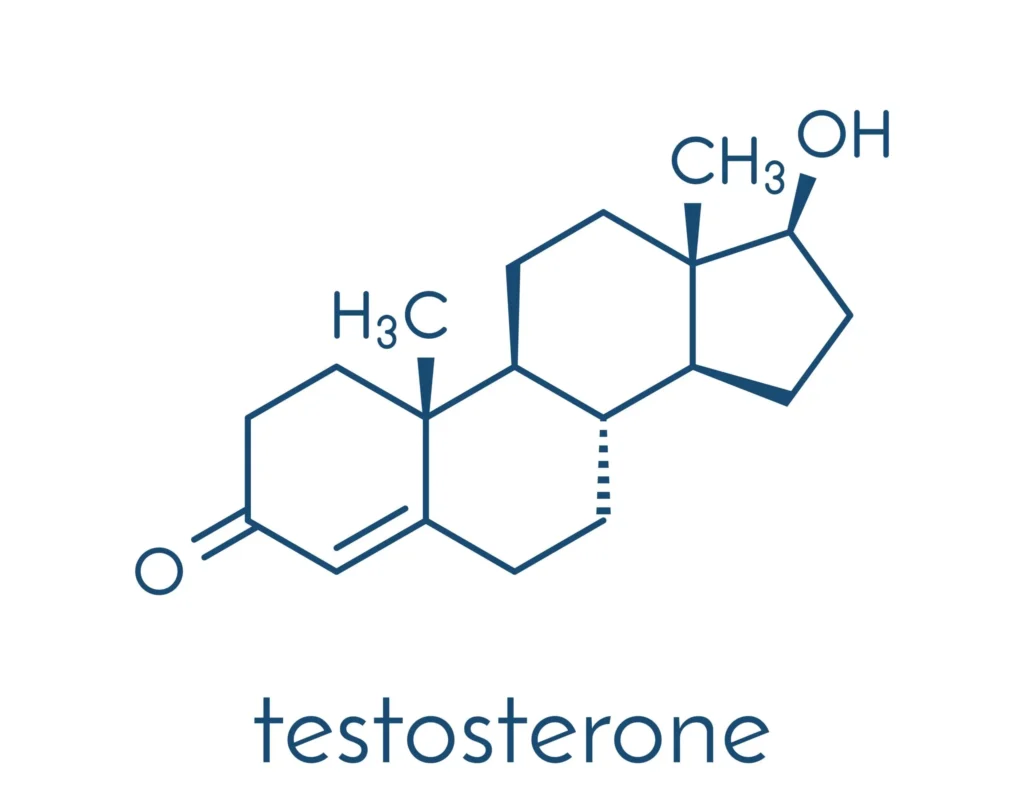
These small but crucial structural features enable testosterone to bind with high affinity to the androgen receptor (AR) and regulate gene transcription.
Mechanism of Action

Androgen Receptor Binding
The androgen receptor (AR) is a nuclear receptor that, upon binding to testosterone or dihydrotestosterone (DHT), translocates to the cell nucleus and initiates a cascade of gene transcription events. While testosterone itself can bind to the androgen receptor, an enzyme called 5α-reductase converts testosterone into DHT in certain tissues like the prostate gland and skin. DHT often has a higher binding affinity for AR, making it a more potent androgen for distinct physiological processes (Goodman & Gilman, “The Pharmacological Basis of Therapeutics,” 13th ed.).
Gene Transcription and Cellular Effects
Once bound to the androgen receptor, the hormone-receptor complex alters the transcription of specific genes that modulate:
- Muscle protein synthesis
- Bone mineralization
- Hair follicle growth
- Sebum production in the skin
- Erythropoiesis (red blood cell production)
Because these effects rely on transcriptional regulation, they tend to manifest more gradually.
Pharmacokinetics
Absorption
Because testosterone undergoes extensive first-pass metabolism when taken orally, pharmaceutical companies have developed special formulations to circumvent this obstacle. Oral methyltestosterone or undecanoate forms can be absorbed via the lymphatic system, but most oral testosterone is inactivated by the liver if not properly formulated.
Common administration routes include:
- Transdermal (patches, gels)
- Intramuscular injections of testosterone esters (e.g., testosterone cypionate, testosterone enanthate)
- Buccal tablets
- Subcutaneous pellets
Distribution
Once in circulation, testosterone is primarily bound to sex hormone-binding globulin (SHBG) and, to a lesser extent, albumin. Only free testosterone or that loosely bound to albumin can readily diffuse into tissues to bind the androgen receptor.
Metabolism
The liver metabolizes testosterone via oxidation, reduction, and conjugation pathways. Key metabolites include androsterone and etiocholanolone, both excreted largely through the kidneys. In target tissues like the prostate, 5α-reductase converts testosterone to DHT, and in adipose tissue, aromatase converts part of testosterone to estradiol.
Excretion
Testosterone and its metabolites are excreted through bile and urine. Glucuronidation and sulfation of steroids enhance water solubility, facilitating excretion. The half-life of testosterone varies depending on the formulation. For instance, testosterone cypionate injected intramuscularly has a half-life of about eight days, while transdermal or buccal forms may require daily or twice-daily administration.
Pharmacodynamics
Anabolic Effects
Testosterone’s anabolic effects make it a crucial hormone for muscle protein synthesis. It promotes the growth and differentiation of skeletal muscle by increasing nitrogen retention and amino acid uptake. Clinical scenarios such as cachexia from chronic illnesses or age-related sarcopenia benefit from testosterone’s anabolic properties. This anabolic action explains its misuse in sports and bodybuilding, where individuals may use larger-than-therapeutic doses to boost muscle mass and performance.
Androgenic Effects
The androgenic properties of testosterone drive the development of primary and secondary male sexual characteristics. These include:
- Penile and scrotal growth during embryonic development.
- Facial, axillary, and pubic hair growth at puberty.
- Voice deepening via effects on the larynx.
Testosterone is also responsible for spermatogenesis in conjunction with FSH, although this process is far more complex and also involves Sertoli cells in the testes.
Additional Physiological Roles
Beyond sexual function and muscle growth, testosterone influences:
- Bone health: By facilitating the retention of calcium and increasing bone mineral density.
- Red blood cell production: Testosterone can stimulate erythropoiesis, thus elevating hematocrit levels.
- Mood and energy levels: Evidence suggests that low testosterone correlates with depression, fatigue, and reduced libido.
Clinical Uses
Hypogonadism
In men with primary or secondary hypogonadism, testosterone replacement can restore normal androgen levels, reversing symptoms like reduced libido, erectile dysfunction, muscle weakness, low bone density, and mood disturbances. The key is to balance exogenous testosterone in a way that reestablishes physiological levels without suppressing endogenous production more than necessary.
Delayed Puberty
For adolescents showing significantly delayed puberty, clinicians sometimes prescribe low-dose testosterone to stimulate the onset of secondary sexual characteristics. This therapy is carefully monitored to prevent premature epiphyseal closure, which could limit growth (Hadley, “Endocrinology,” 7th ed.).
Gender-Affirming Hormone Therapy
Individuals undergoing gender transition from female to male (FTM) often receive testosterone therapy to promote masculinization (e.g., increased facial and body hair, deeper voice). Long-term testosterone administration must be monitored for both efficacy and adverse effects.
Other Therapeutic Uses
• Anabolic deficiencies: Certain chronic illnesses like HIV/AIDS or end-stage renal disease can lead to significant muscle wasting; testosterone supplementation may mitigate this.
• Improving bone density: Postmenopausal women with osteoporosis sometimes benefit from low-dose androgen therapy alongside estrogen, though this is less common.
• Male contraception (investigational): Efforts are ongoing to create an androgen-based contraceptive to suppress spermatogenesis. Early research has highlighted the complexity of balancing gonadotropin suppression with the need to maintain normal physical and metabolic functions.
Testosterone Replacement Therapy (TRT)
Rationale for TRT
Testosterone Replacement Therapy (TRT) aims to restore physiological hormone levels in individuals deficient in endogenous testosterone. Aging men often experience andropause, characterized by gradually falling testosterone levels, though the exact relationship with clinical symptoms is debated.
Formulations
Common TRT formulations include:
- Injectable esters: Such as testosterone enanthate, testosterone cypionate, and testosterone propionate.
- Transdermal patches or gels: Provide steady testosterone release while reducing hepatic metabolism; require daily application.
- Transbuccal systems: Placed above the incisor tooth, offering a noninvasive alternative.
- Oral capsules (testosterone undecanoate): Absorbed via the lymphatic system but subject to variable bioavailability.
Monitoring and Dose Adjustment
Clinicians monitor serum testosterone, estradiol, hematocrit, and prostate-specific antigen (PSA) levels periodically when initiating TRT. Adjustments in dosage or formulation may follow if patients experience side effects such as elevated hematocrit or if they do not adequately respond to therapy (Bennett & Brown, “Clinical Pharmacology,” 12th ed.).
Adverse Effects
While testosterone therapy can be transformative for individuals with low levels, it carries certain risks:
- Cardiovascular Risks: Some studies have linked testosterone supplementation with increased risk of thrombotic events in predisposed individuals. However, the data are mixed, and more research is needed.
- Erythrocytosis: Testosterone enhances erythropoiesis, potentially leading to elevated hematocrit. This can increase blood viscosity and risk of thrombosis. Regular hematocrit monitoring is thus essential.
- Suppression of Spermatogenesis: Exogenous testosterone reduces GnRH and subsequently FSH, impairing sperm production and potentially leading to infertility.
- Gynecomastia: Peripheral aromatization of testosterone to estradiol can cause breast tissue development in men.
- Acne and Oily Skin: Androgens stimulate sebaceous gland activity, which can increase acne.
- Mood Changes: Individuals may experience mood swings, irritability, or aggression when exposed to supra-physiological doses.
In older males with underlying prostatic disease, benign prostatic hyperplasia (BPH) may worsen, and there is a theoretical concern about accelerating prostate cancer in susceptible individuals.
Contraindications
Testosterone therapy is contraindicated or requires extreme caution in:
- Known or suspected prostate cancer
- Male breast cancer
- Uncontrolled congestive heart failure
- Severe obstructive sleep apnea
- Hematocrit >50%
Risk-versus-benefit analyses remain crucial in cases with multiple comorbidities. Comprehensive assessment, including PSA testing and digital rectal examination, is standard before initiating testosterone therapy.
New Developments in Testosterone Therapy
Novel Formulations
Recent years have seen the development of long-acting injectables like testosterone undecanoate designed for administration every 10 to 14 weeks, improving patient compliance. Additionally, subcutaneous and nasal formulations are under study to offer more convenient administration routes.
Selective Androgen Receptor Modulators (SARMs)
SARMs aim to preserve the beneficial anabolic actions on muscle and bone while minimizing the androgenic effects on tissues like the prostate. Though SARMs remain in experimental stages, they may represent the next generation of therapy for both hypogonadism and age-related muscle wasting.
Gene Editing and Cellular Therapies
Future modalities may include gene editing approaches intended to correct enzymatic defects in the testosterone biosynthetic pathway or to modulate androgen receptor activity directly. While these are still largely theoretical, they offer a glimpse into the potential for personalized and targeted hormonal therapies (Rang & Dale, “Rang and Dale’s Pharmacology,” 9th ed.).
Conclusion
The pharmacology of testosterone spans endocrinology, metabolism, and reproductive medicine, underscoring the hormone’s multifaceted role in health and disease. From its early identification in the 1930s to the advent of new therapeutic strategies, testosterone has been a cornerstone of male reproductive physiology and a valuable tool in the treatment of multiple clinical conditions, including hypogonadism, delayed puberty, and certain forms of muscle wasting.
Key factors in testosterone management include: • The interplay of the hypothalamic-pituitary-gonadal (HPG) axis
• Careful formulation selection for bioavailability and patient convenience
• Rigorous safety monitoring to mitigate risks such as erythrocytosis, gynecomastia, and prostate pathology
Looking ahead, researchers and clinicians will continue to refine the efficacy and safety of testosterone therapy. Emerging technologies, such as SARMs and even potential gene-based approaches, may revolutionize how we treat androgen-deficient conditions, bringing personalized medicine ever closer to reality. Monitoring ongoing clinical trials and regulatory developments remains essential for healthcare practitioners and patients alike.
Ultimately, testosterone’s clinical significance extends beyond its role in shaping male characteristics; it is a hormone intimately tied to bone health, erythropoiesis, metabolic homeostasis, and psychological well-being. By mastering its pharmacology, clinicians can optimize treatments, enhance patient quality of life, and continue to push the boundaries of hormone research.
References
- Bennett, P. N., & Brown, M. J. (2012). Clinical Pharmacology (12th ed.). Elsevier.
- Goodman, L. S., & Gilman, A. G. (2017). The Pharmacological Basis of Therapeutics (13th ed.). McGraw-Hill.
- Guyton, A. C., & Hall, J. E. (2016). Textbook of Medical Physiology (13th ed.). Elsevier.
- Hadley, M. E. (2016). Endocrinology (7th ed.). Pearson.
- Katzung, B. G., & Trevor, A. (2018). Basic and Clinical Pharmacology (14th ed.). McGraw-Hill.
- Rang, H. P., & Dale, M. M. (2019). Rang and Dale’s Pharmacology (9th ed.). Elsevier.
Medical Disclaimer
The medical information on this post is for general educational purposes only and is provided by Pharmacology Mentor. While we strive to keep content current and accurate, Pharmacology Mentor makes no representations or warranties, express or implied, regarding the completeness, accuracy, reliability, suitability, or availability of the post, the website, or any information, products, services, or related graphics for any purpose. This content is not a substitute for professional medical advice, diagnosis, or treatment; always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition and never disregard or delay seeking professional advice because of something you have read here. Reliance on any information provided is solely at your own risk.

