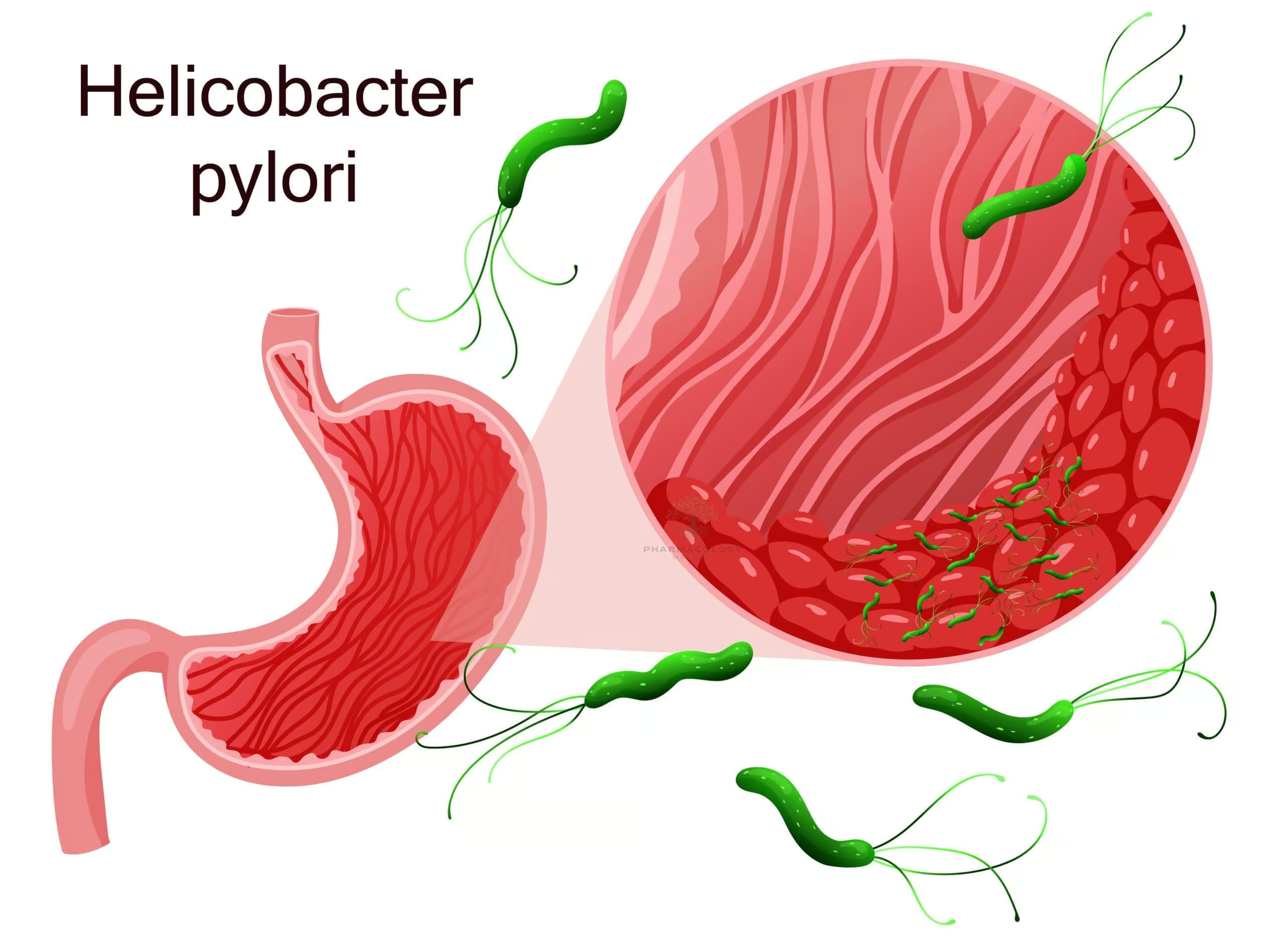
Introduction
Helicobacter pylori (H. pylori) is a spiral-shaped, gram-negative bacterium that colonizes the human stomach and duodenum. First identified in the early 1980s, this organism quickly garnered attention due to its critical role in the pathogenesis of peptic ulcer disease, chronic gastritis, and certain types of gastric malignancies, notably gastric adenocarcinoma and mucosa-associated lymphoid tissue (MALT) lymphoma. Despite significant advances in our understanding of H. pylori, it remains a prevalent global health concern, infecting roughly half of the world’s population. Transmission is believed to primarily occur via the fecal-oral or oral-oral routes, often reflecting socioeconomic factors such as water sanitation and living conditions.
Identification of H. pylori revolutionized the management of peptic ulcer disease, which had historically relied on acid suppression therapy without addressing the underlying infection. Modern treatment strategies aim to eradicate H. pylori to prevent ulcer recurrence and reduce the risk of gastric cancer development. This article provides an overview of the epidemiology, pathophysiology, clinical manifestations, diagnostic tools, and the latest treatment modalities for H. pylori infection.
Epidemiology
H. pylori infection rates vary globally based on age, region, and socioeconomic status. In general, the bacterium is more prevalent in resource-limited settings, reflecting higher rates of crowding, poor hygiene, and limited access to clean water. Infection commonly begins in childhood, and without treatment, it can persist throughout life. As living standards improve, the overall prevalence of H. pylori tends to decline. Nevertheless, even in high-income countries, pockets of higher infection rates can exist, especially among older populations and specific ethnic or immigrant communities.
Pathophysiology and Virulence Factors
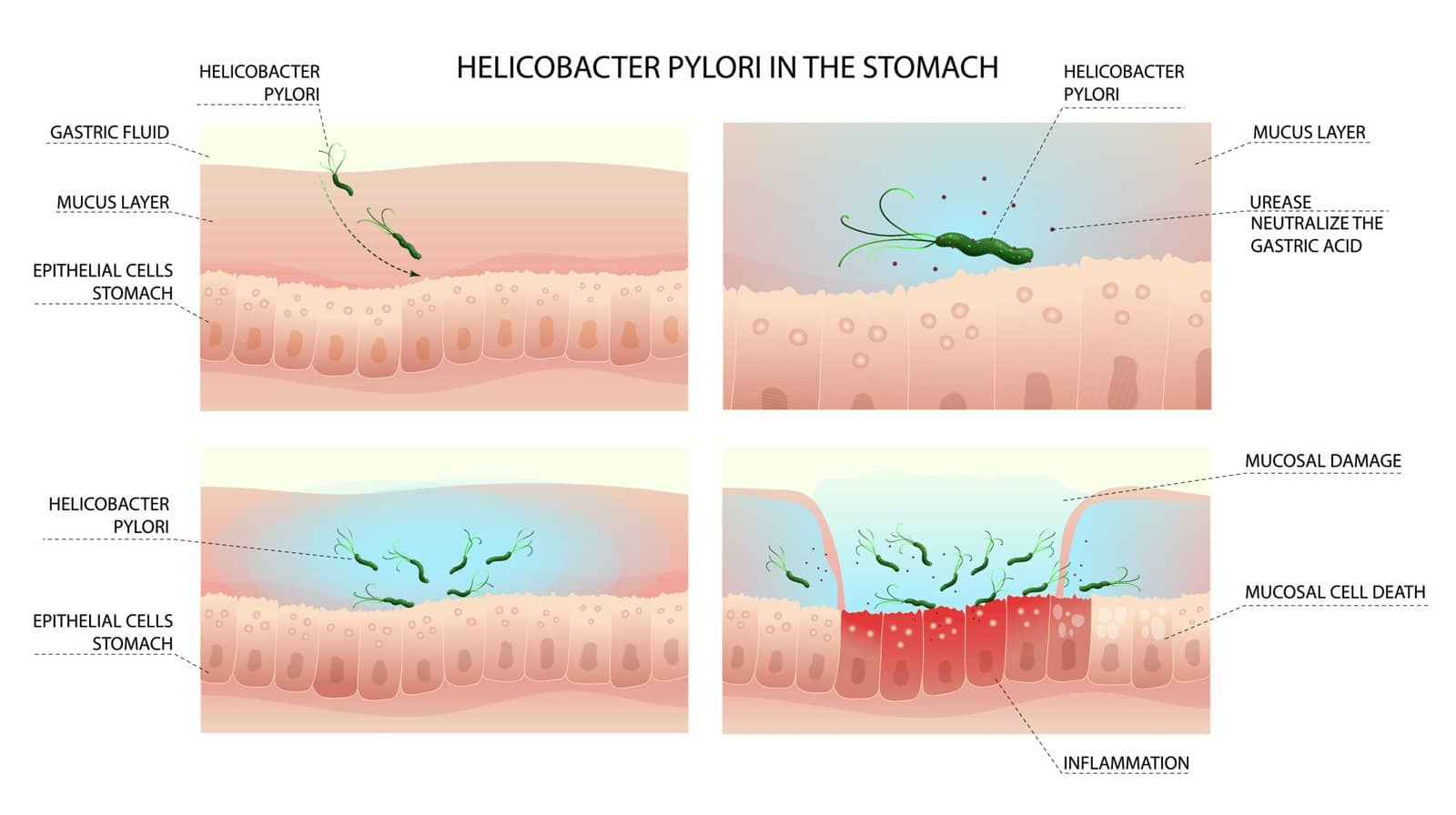
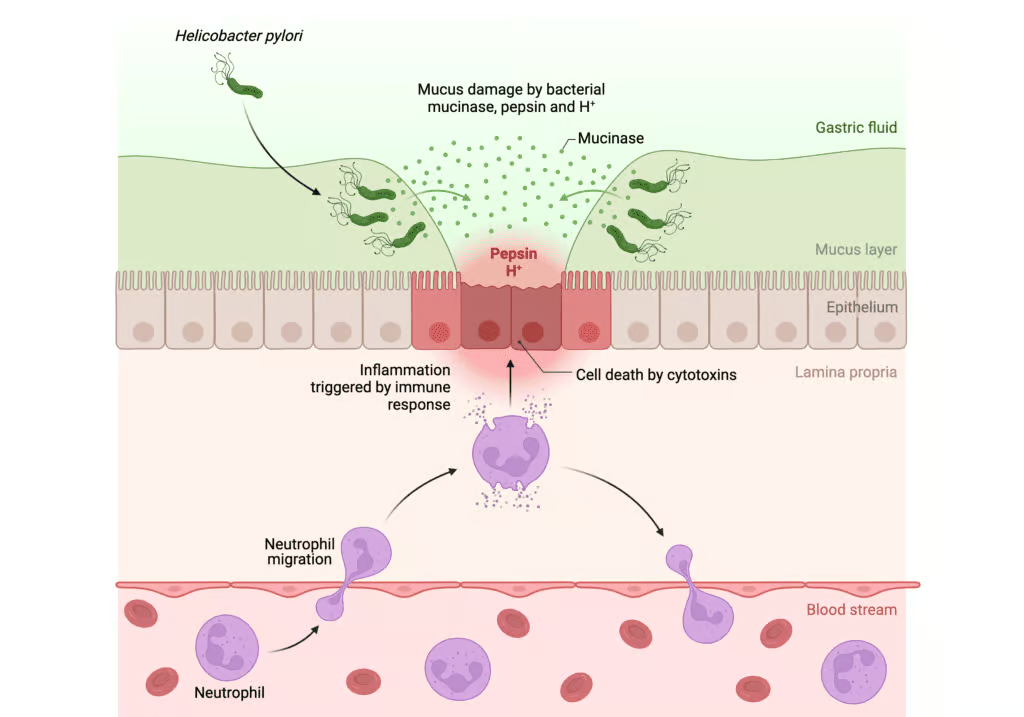
Once ingested, H. pylori traverses the gastric lumen to settle within the thick mucus layer that coats the stomach. This environment, while hostile to many bacteria, is made tolerable for H. pylori by several adaptive mechanisms:
- Urease Production: The bacterium produces urease, an enzyme that hydrolyzes urea (present in the stomach) into ammonia and carbon dioxide. Ammonia neutralizes local gastric acid, creating a more protective microenvironment for the organism.
- Flagella: H. pylori bears multiple flagella, enabling it to penetrate the viscous mucus layer and adhere to gastric epithelial cells.
- Adhesins and Toxins: Surface adhesins allow the bacteria to attach to epithelial cells. Certain strains produce cytotoxin-associated gene A (CagA) and vacuolating cytotoxin A (VacA), which can induce inflammation, cell damage, and apoptosis.
Upon colonization, H. pylori orchestrates a complex immune response, causing chronic active gastritis. This inflammation may remain asymptomatic or progress to peptic ulcers, gastric atrophy, intestinal metaplasia, and—in a subset of patients—malignancy. Genetic predisposition, environmental factors, and specific bacterial virulence determinants all contribute to disease severity and outcomes.
Clinical Manifestations
While many people remain asymptomatic for years or decades, a significant proportion develop gastrointestinal symptoms, including:
- Peptic Ulcer Disease: H. pylori is responsible for the majority of duodenal and a considerable proportion of gastric ulcers. Patients may present with epigastric pain, dyspepsia, or, in some cases, bleeding manifesting as melena or hematemesis.
- Chronic Gastritis: Persistent infection leads to chronic active gastritis, which may or may not cause significant clinical symptoms.
- Gastric Adenocarcinoma: Chronic inflammatory changes over time can promote a sequence from atrophic gastritis to intestinal metaplasia and eventually malignant transformation in certain patients.
- MALT Lymphoma: H. pylori infection is strongly associated with gastric MALT lymphoma. Remarkably, eradication of the organism can lead to remission of early-stage MALT lymphoma in many cases.
Although the majority of H. pylori–positive individuals may only experience subclinical or mild dyspeptic symptoms, vigilance is essential for early recognition of complications.
Diagnosis
Several modalities exist to diagnose H. pylori, and selection often depends on the clinical scenario and availability of tests:
- Non-Invasive Tests
- Urea Breath Test (UBT): Patients ingest labeled urea (either carbon-13 or carbon-14). If H. pylori is present, urease cleaves the urea, releasing labeled carbon dioxide detectable in the patient’s exhalation.
- Stool Antigen Test: Detects H. pylori antigens in fecal samples and is well-suited for both diagnosis and for confirming eradication after therapy.
- Serology: While it indicates exposure to H. pylori, it does not distinguish between active and past infection. Consequently, serology is used less frequently for diagnostic confirmation.
- Invasive Tests (Endoscopic)
- Biopsy-Urease Test (Rapid Urease Test): Gastric biopsy specimens are placed in a medium containing urea and a pH indicator. A color change indicates the presence of H. pylori.
- Histology: Direct microscopic visual inspection of stained gastric biopsy tissues.
- Culture: Gastric biopsy is cultured for H. pylori, allowing antimicrobial susceptibility testing—a critical step in treatment-resistant or complex cases.
For initial diagnosis, non-invasive tests (e.g., UBT or stool antigen test) are often preferred. Endoscopy is typically reserved for patients with alarm symptoms, older adults, or those requiring evaluation for complications like ulcers or malignancy.
Treatment Rationale and Guiding Principles
H. pylori treatment aims to eradicate the pathogen, thereby providing ulcer healing, preventing recurrence, and mitigating the risk of gastric malignancies. An effective regimen must incorporate agents that reduce gastric acidity (to create a more hospitable environment for antibiotics), combined with antibiotics active against H. pylori. However, rising global antibiotic resistance poses challenges to achieving high eradication rates.
General principles in choosing a therapeutic regimen include
- Using a proton pump inhibitor (PPI) to raise gastric pH;
- Combining two or more antibiotics to decrease the likelihood of resistance; and
- Ensuring adequate course duration (commonly 10-14 days, though regimens of 14 days are increasingly recommended to maximize efficacy).
Following completion of therapy, a test of cure (usually a urea breath test or stool antigen test) is recommended at least four weeks after antibiotics and two weeks after stopping PPIs to confirm successful eradication.
Currently Recommended Treatment Regimens
- Triple Therapy (though it isfalling out of favor in areas with high clarithromycin resistance)
- A PPI (e.g., omeprazole, lansoprazole)
- Clarithromycin
- Amoxicillin (or metronidazole in penicillin-allergic individuals)
- Duration: 14 days preferred over shorter courses.
- Bismuth Quadruple Therapy
- A PPI
- Bismuth subsalicylate or subcitrate
- Tetracycline
- Metronidazole
- Duration: 10-14 days
This regimen is commonly used in areas with high clarithromycin resistance or in patients who fail first-line triple therapy.
- Concomitant Therapy
- A PPI
- Clarithromycin
- Amoxicillin
- Metronidazole (or tinidazole)
- Duration: 10-14 days
This regimen differs from classic triple therapy by including metronidazole or tinidazole in addition to the standard triple therapy components, thereby broadening antibiotic coverage.
- Sequential or Hybrid Therapy
- Sequential Therapy involves taking a PPI plus amoxicillin for the initial five to seven days, followed by a PPI, clarithromycin, and metronidazole or tinidazole for the next five to seven days.
- Hybrid Therapy combines aspects of sequential and concomitant therapy.
- Levofloxacin-containing Regimens
In patients who fail initial therapy or in areas with high levels of clarithromycin resistance, levofloxacin may replace clarithromycin. While effective, resistance to fluoroquinolones is also on the rise, underscoring the importance of culture and sensitivity testing in difficult cases.
Key Consideration: Local antibiotic resistance patterns heavily shape treatment selection. Monitoring of eradication rates and adaptation of guidelines at local and regional levels help optimize success.
Resistance Concerns and Salvage Therapy
Antibiotic resistance is a major threat to successful H. pylori treatment. Clarithromycin resistance, in particular, is a leading cause of treatment failure. Bismuth quadruple therapy offers an effective alternative in cases where clarithromycin resistance is high or suspected. If first-line therapy fails, salvage therapy—often employing a different antibiotic combination—should be attempted. Antibiotic sensitivity testing via endoscopy and culture can guide subsequent choices, ensuring that the regimen targets the organism effectively.
Special Patient Populations
- Children: Pediatric regimens parallel adult recommendations, but medication tolerability and formulation availability can limit options. The duration and dosing may be adjusted based on age and weight.
- Pregnant or Lactating Women: Tetracyclines and fluoroquinolones are typically contraindicated. PPI safety profiles should be considered, and clarithromycin, though Category C, may be used cautiously if benefits outweigh risks.
- Penicillin Allergy: Metronidazole can substitute for amoxicillin; however, if the patient also has resistance to macrolides, bismuth quadruple therapy often becomes necessary.
Prevention and Outlook
Public health efforts aimed at improving hygiene, water quality, and living standards can reduce H. pylori incidence. Vaccines under development could further decrease new infections, though none are currently commercially available. As research continues to refine therapeutic regimens, key focuses include curbing antibiotic resistance, optimizing eradication rates, and ensuring safe, cost-effective approaches for varied populations.
H. pylori eradication remains critical in adults with confirmed infection who also have peptic ulcer disease or a history of ulcers. Eradication is also recommended for patients with gastric MALT lymphoma, atrophic gastritis, partial gastric resections for early gastric cancer, or in those with first-degree relatives who have had gastric cancer. Additionally, individuals with refractory dyspepsia or chronic gastritis may benefit from eradication, according to consensus guidelines.
Conclusion
The discovery of H. pylori fundamentally transformed our understanding and management of peptic ulcer disease and gastritis. An adaptable bacterium, H. pylori persists as a significant public health concern due to its remarkable prevalence and association with ulcer formation and gastric malignancies. Timely and appropriate eradication strategies offer profound clinical benefits, preventing future ulcer recurrence, reducing gastric cancer risk, and alleviating chronic dyspeptic symptoms for many patients.
With antibiotic resistance on the rise, adherence to evidence-based regimens, consideration of local resistance patterns, and follow-up testing to confirm eradication are all essential components of effective care. Ongoing research into innovative therapies and a potential vaccine may further bolster our capacity to control and eradicate H. pylori, improving global gastric health outcomes.
References
- Feldman M, Friedman LS, Brandt LJ, editors. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. 10th ed. Philadelphia: Elsevier Saunders; 2016.
- Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection—the Maastricht V/Florence Consensus Report. Gut. 2017;66(1):6-30.
- Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1(8390):1311-1315.
- Hunt RH, Camilleri M, Crowe SE, et al. The stomach and duodenum. In: Kasper DL, Fauci AS, Hauser SL, Longo DL, Jameson JL, Loscalzo J, editors. Harrison’s Principles of Internal Medicine. 20th ed. New York: McGraw-Hill; 2018. p. 2215-2232.