Fluoroquinolones are one of the most significant classes of broad-spectrum antimicrobial agents used in modern medicine. With their ability to target both Gram-positive and Gram-negative bacteria, these agents are frequently employed in various clinical scenarios, from community-acquired respiratory infections to complicated hospital-acquired pathogens. Developed from the original compound nalidixic acid in the 1960s, fluoroquinolones have evolved to exhibit increased potency, improved pharmacokinetics, and a broader coverage. Despite their therapeutic benefits, caution is warranted due to adverse effects and the potential for bacterial resistance.
In this comprehensive article, we will delve into the pharmacology of fluoroquinolones, guided by reputed pharmacology textbooks such as Goodman & Gilman’s The Pharmacological Basis of Therapeutics, Basic & Clinical Pharmacology by Katzung, and Rang & Dale’s Pharmacology. Topics include their history, structure, classification, mechanism of action, pharmacokinetics, pharmacodynamics, clinical applications, adverse effects, resistance patterns, and future directions. By blending authoritative references with practical pharmacological insights, this article aims to serve as a valuable resource for healthcare professionals, students, and researchers.
1. Historical Background and Development
The quinolone class of antimicrobials originated with nalidixic acid, discovered as a byproduct of chloroquine synthesis in the early 1960s. Although nalidixic acid provided a template for urinary tract infection therapies, its limited spectrum propelled researchers to seek more potent analogs. The addition of a fluorine atom at the C-6 position of the quinolone nucleus marked the advent of fluoroquinolones, exemplified by norfloxacin in the late 1970s.
Subsequent generations of fluoroquinolones emerged as medicinal chemists further refined the quinolone nucleus to enhance Gram-positive coverage, bioavailability, tissue penetration, and pharmacokinetic profiles. Agents like ciprofloxacin, ofloxacin, and levofloxacin represented quantum leaps in efficacy. Later developments, such as moxifloxacin and gemifloxacin, offered improved activity against respiratory pathogens. Over the decades, fluoroquinolones transformed into a cornerstone antibiotic class. However, concerns about antimicrobial resistance and adverse effects necessitate judicious use.
2. Chemical Structure and Classification
Fluoroquinolones are derived from the quinoline carboxylic acid framework. Key structural features include:
- A fluorine atom at position 6, enhancing antimicrobial potency.
- Substituents at position 1 and position 7 that influence spectrum and pharmacokinetics.
These modifications have given rise to various “generations” of fluoroquinolones:
- First-Generation: E.g., nalidixic acid (technically a quinolone, not fluoroquinolone) focusing mainly on Gram-negative coverage in the urinary tract.
- Second-Generation: E.g., ciprofloxacin, norfloxacin, and ofloxacin exhibit enhanced Gram-negative coverage (including Pseudomonas), with moderate Gram-positive activity.
- Third-Generation: E.g., levofloxacin, with better Gram-positive coverage, particularly against Streptococcus pneumoniae.
- Fourth-Generation: E.g., moxifloxacin, gemifloxacin, recognized for improved Gram-positive and atypical pathogen coverage (including activity against anaerobes in certain cases).
Though “generation” terminology is imprecise, it offers a simplified understanding of spectrum evolution. Specific chemical changes in ring substituents continue to refine pharmacological profiles.
3. Mechanism of Action
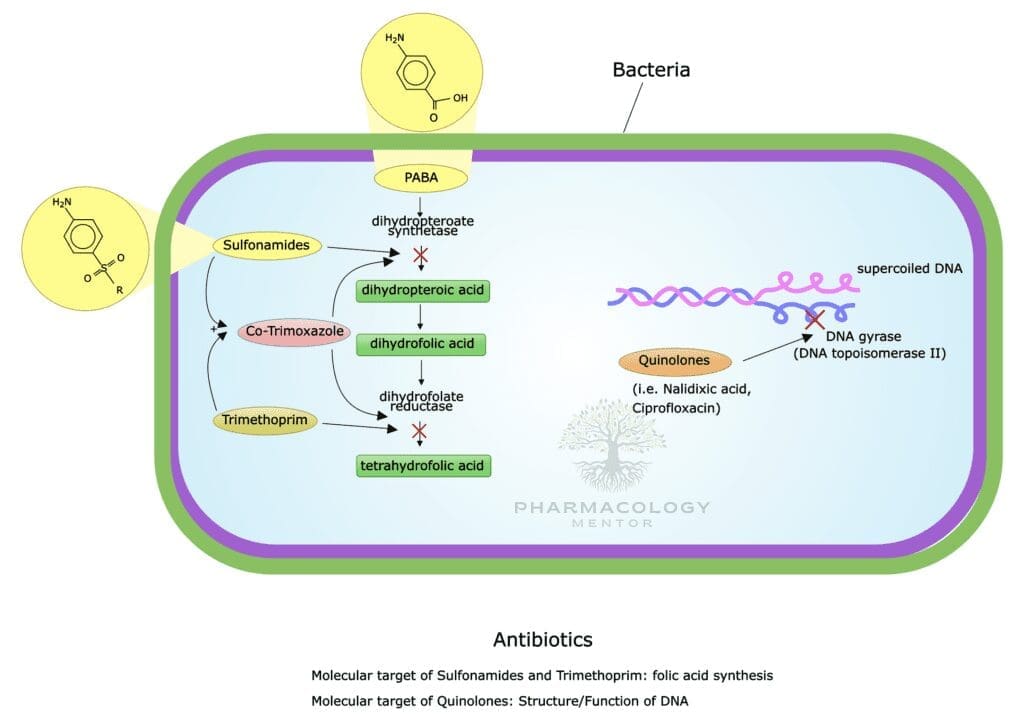
Fluoroquinolones primarily target bacterial DNA gyrase (topoisomerase II) in Gram-negative organisms and topoisomerase IV in Gram-positive organisms. These topoisomerases manage DNA supercoiling and segregation during bacterial replication. By inhibiting these enzymes, fluoroquinolones:
- Induce DNA strand breaks,
- Hinder bacterial replication,
- Lead to cell death (bactericidal effect).
Concentration-dependent killing characterizes fluoroquinolones: higher blood levels correlate with enhanced bacterial killing rates. According to Goodman & Gilman’s, adequate peak concentration relative to the organism’s minimum inhibitory concentration (MIC) drives the therapeutic success.
4. Pharmacokinetics of Fluoroquinolones
A key strength of fluoroquinolones lies in their favorable pharmacokinetic properties, allowing for flexible routes of administration, including oral and intravenous:
- Absorption
- Most fluoroquinolones boast high oral bioavailability, often exceeding 70–80%.
- Foods and gastric acidity have limited impact on absorption (although certain metals like calcium, iron, magnesium, or zinc can chelate fluoroquinolones, reducing their absorption).
- Distribution
- Fluoroquinolones distribute extensively across tissues and achieve high intracellular concentrations, important for targeting intracellular pathogens (e.g., Chlamydia, Mycobacteria).
- Tissue penetration into the prostate, urinary tract, lungs, and bone is strong, making them especially valuable for infections in these sites.
- Metabolism
- Extent of hepatic metabolism varies. Ciprofloxacin undergoes partial metabolism, whereas moxifloxacin is more extensively metabolized in the liver.
- The resultant metabolites may or may not retain antibacterial activity, depending on the specific fluoroquinolone.
- Excretion
- Renal excretion is common, often in the range of 30–60% of the administered dose.
- Some agents (e.g., moxifloxacin) undergo biliary excretion, adding further utility in intra-abdominal infections and hepatic clearance.
- Dose adjustments for renal impairment may be required with specific fluoroquinolones (e.g., ciprofloxacin, levofloxacin).
- Half-Life
- The half-life typically ranges from 3–10 hours. Longer half-lives (e.g., levofloxacin and moxifloxacin) facilitate once-daily dosing regimens.
- Pharmacokinetic-Pharmacodynamic (PK-PD) Considerations
- Concentration-dependent killing: Achieving high serum levels (peak/MIC ratio) is more critical than total time above the MIC, guiding once-daily or high-dose regimens.
- Area Under the Concentration-Time Curve (AUC)/MIC ratio is another critical parameter, correlating with microbiological success.
5. Spectrum of Activity
Fluoroquinolones differ in their coverage, but collectively, they exhibit a broad spectrum:
- Gram-Negative Bacteria
- Most fluoroquinolones excel at inhibiting Enterobacteriaceae (e.g., E. coli, Klebsiella species, Proteus species).
- They exhibit potent activity against Pseudomonas aeruginosa, especially ciprofloxacin, making fluoroquinolones valuable in hospital settings for pseudomonal infections.
- Gram-Positive Bacteria
- Earlier fluoroquinolones had limited efficacy against Gram-positive organisms. Newer agents (e.g., levofloxacin, moxifloxacin) have improved coverage, especially against Streptococcus pneumoniae (including certain resistant strains).
- They may have moderate activity against Staphylococcus aureus, but rising resistance, especially MRSA, limits clinical utility.
- Atypical Pathogens
- Legionella pneumophila, Chlamydophila pneumoniae, Mycoplasma pneumoniae are effectively targeted, making respiratory fluoroquinolones a mainstay in community-acquired pneumonia (CAP).
- Anaerobes
- Older fluoroquinolones (e.g., ciprofloxacin) typically lack robust anaerobic coverage.
- Moxifloxacin and delafloxacin provide better anaerobic activity, beneficial in mixed infections (e.g., intra-abdominal, diabetic foot infections).
- Other Pathogens
- Some fluoroquinolones have mycobacterial coverage, making them adjunct therapies for tuberculosis or nontuberculous mycobacteria.
6. Clinical Applications and Therapeutic Uses
Fluoroquinolones’ broad coverage and excellent tissue penetration underpin their utility in various infectious disease scenarios:
- Respiratory Tract Infections
- Levofloxacin and moxifloxacin are sometimes referred to as “respiratory fluoroquinolones.”
- They effectively target typical CAP pathogens, including Streptococcus pneumoniae, and atypical organisms (Chlamydophila, Mycoplasma, Legionella).
- Indicated for community-acquired pneumonia, acute exacerbations of chronic bronchitis, and sometimes hospital-acquired pneumonia.
- Urinary Tract Infections
- Ciprofloxacin and levofloxacin remain cornerstones for complicated UTIs and pyelonephritis, primarily due to robust activity against E. coli and other Enterobacteriaceae, plus favorable renal excretion.
- However, emerging resistance has sometimes reduced their efficacy in uncomplicated UTIs.
- Gastrointestinal Infections
- Fluoroquinolones can be used to treat traveler’s diarrhea, including infections by enterotoxigenic E. coli.
- In combination with metronidazole or imidazole derivatives, they can address intra-abdominal infections caused by Gram-negative rods and anaerobes.
- Skin and Soft Tissue Infections
- Fluoroquinolones (particularly newer-generation ones) offer coverage for various pathogens implicated in skin infections, though MRSA coverage is unreliable.
- Mixed infections (Gram-negative, some anaerobes) may benefit from broad-spectrum coverage.
- Bone and Joint Infections
- Good penetration into bone supports occasional use in osteomyelitis (e.g., due to Gram-negative bacilli).
- Oral fluoroquinolones can sometimes be used for step-down therapy from IV medications.
- Sexually Transmitted Infections (STIs)
- Although not first-line in gonorrhea due to rising resistance, fluoroquinolones once played a major role.
- They retain activity against Chlamydia but under most guidelines, tetracyclines or macrolides are typically favored.
- Prophylaxis
- Neutropenic patients or those undergoing certain invasive procedures might receive a fluoroquinolone for infection prophylaxis, owing to potent Gram-negative coverage.
- Ophthalmic and Otic Preparations
- Several fluoroquinolones (e.g., ofloxacin, ciprofloxacin) are formulated as eyedrops or ear drops to manage local infections with minimal systemic exposure.
7. Adverse Effects and Toxicities
While fluoroquinolones are generally well-tolerated, clinicians must remain alert to potential adverse reactions:
- Gastrointestinal (GI) Disturbances
- Nausea, vomiting, diarrhea, and abdominal pain represent the most common side effects.
- Risk of Clostridioides difficile infection is present, though not as pronounced as with certain β-lactams or clindamycin.
- Central Nervous System (CNS) Effects
- Headache, dizziness, confusion, or even seizures can arise, especially with higher doses or in patients with reduced renal function.
- Fluoroquinolones may lower the seizure threshold by antagonizing GABA receptors (particularly notable with ciprofloxacin).
- Tendon Damage and Musculoskeletal Effects
- Tendonitis and tendon rupture (especially the Achilles tendon) are serious complications.
- Patients over 60 years old, those receiving corticosteroids, or post-transplant recipients are at higher risk.
- The FDA currently recommends caution when prescribing fluoroquinolones for mild infections in these high-risk groups.
- Cardiac Effects
- Fluoroquinolones can prolong the QT interval, heightening the risk of arrhythmias (e.g., Torsade de Pointes), especially in patients on other QT-prolonging medications.
- Moxifloxacin is particularly associated with QT prolongation.
- Neuromuscular Blockade
- Fluoroquinolones can exacerbate muscle weakness in patients with myasthenia gravis, potentially precipitating respiratory failure.
- Hypo- or Hyperglycemia
- Dysglycemia, especially in diabetic patients.
- Gatifloxacin was particularly notorious for this but is now less commonly used in many regions.
- Photosensitivity
- Although more typical of older agents, patients may experience an increased sensitivity to sunlight or UV light, warranting protective measures.
- Potential Aortic Aneurysm and Dissections
- Emerging data have suggested a possible association between fluoroquinolone use and an increased risk of aortic dissections and aneurysms, prompting regulatory warnings and prescribing caution.
8. Mechanisms of Bacterial Resistance
The overuse and misuse of fluoroquinolones have significantly contributed to resistance development in bacteria worldwide. Bacterial pathogens adopt multiple resistance mechanisms:
- Mutation in Target Enzymes
- Mutations in DNA gyrase (GyrA or GyrB subunits) or topoisomerase IV genes reduce drug binding affinity.
- A single point mutation may confer a moderate decrease in susceptibility; additional mutations amplify resistance levels.
- Efflux Pumps
- Bacteria may upregulate or acquire efflux pumps, actively pumping fluoroquinolones out of the cell, lowering intracellular concentrations.
- Reduced Permeability
- Gram-negative bacteria can decrease porin channels in their outer membrane, inhibiting antibiotic entry.
- Plasmid-Mediated Resistance
- Genes encoding Qnr proteins or modified aminoglycoside acetyltransferases can be transferred horizontally, conferring partial protection against fluoroquinolones.
Resistance issues are most pronounced among Pseudomonas aeruginosa, Acinetobacter, and certain Enterobacteriaceae (e.g., E. coli with mutated gyrase). In many regions, rising resistance has led guidelines to discourage fluoroquinolones as first-line therapy for uncomplicated infections.
9. Drug Interactions
Fluoroquinolones can interact with other drugs or substances in various ways:
- Metals and Antacids
- As mentioned, chelates formed with calcium, magnesium, aluminum, or iron can heavily reduce bioavailability.
- Patient counseling should emphasize spacing the ingestion of fluoroquinolones and mineral-containing supplements by at least 2 hours.
- Theophylline and Caffeine
- Certain fluoroquinolones, notably ciprofloxacin, can inhibit the hepatic metabolism (CYP1A2) of theophylline and caffeine, increasing their levels and potential toxicity.
- Warfarin
- Enhanced anticoagulant effects may be seen, due to alterations in vitamin K-producing gut flora or potential partial metabolic interference, warranting close INR monitoring.
- QT-Prolonging Drugs
- Co-administration with other QT-prolonging agents (e.g., certain antipsychotics, antiarrhythmics, macrolides) amplifies arrhythmia risks, necessitating ECG monitoring.
- Oral Hypoglycemics / Insulin
- Dysglycemia potential calls for vigilance in diabetic patients on antidiabetic regimens.
10. Specific Agents and Their Distinct Properties
- Ciprofloxacin
- Spectrum: Strong Gram-negative coverage (including Pseudomonas), moderate Gram-positive activity.
- Uses: UTIs, intra-abdominal infections, Pseudomonas pneumonia, prophylaxis for anthrax (Bacillus anthracis).
- Notes: Well-known for drug interactions with theophylline and caffeine; can have significant CNS side effects.
- Levofloxacin
- Spectrum: Enhanced Gram-positive coverage (particularly S. pneumoniae), retains good Gram-negative activity.
- Uses: Respiratory infections, UTIs, skin infections.
- Notes: “Respiratory fluoroquinolone”; generally well-tolerated, but QT prolongation risk is present.
- Moxifloxacin
- Spectrum: Excellent coverage for Gram-positive and atypical organisms, plus some anaerobes. Less activity against Pseudomonas.
- Uses: Community-acquired pneumonia (CAP), intra-abdominal infections in combination therapy.
- Notes: More extensive hepatic metabolism; associated with a higher risk of QT prolongation.
- Gemifloxacin
- Spectrum: Potent antipseudomonal activity is modest; primarily used for respiratory infections.
- Uses: CAP and acute bacterial exacerbations of chronic bronchitis.
- Notes: Watch for rashes in younger women and modest coverage for Gram-negative pathogens.
- Delafloxacin
- Spectrum: Improved MRSA coverage; robust against Gram-negatives.
- Uses: Skin and soft tissue infections (SSTIs), with FDA approval for community-acquired pneumonia.
- Notes: Emerging as a newer-generation agent with expanded coverage.
11. Role in Antimicrobial Stewardship
Concerns about fluoroquinolone resistance and adverse effects culminated in new monitoring by international regulatory agencies (such as the U.S. FDA and the European Medicines Agency) and professional organizations (e.g., Infectious Diseases Society of America, IDSA). Key stewardship considerations:
- Narrower-Spectrum Alternatives First
- Infections readily managed by alternative antibiotics (e.g., nitrofurantoin for uncomplicated cystitis) should not default to fluoroquinolones.
- Restricting Use
- Hospitals often track fluoroquinolone prescription volumes to reduce overexposure and slow the development of resistance.
- De-Escalation when Possible
- Empiric broad coverage might be narrowed or switched to a safer/more targeted agent when culture results become available.
- Education
- Prescribers must be aware of potential toxicities (e.g., tendon rupture, QT prolongation, CNS effects) and weigh risks vs. benefits.
- Duration of Therapy
- Minimizing the length of antibiotic courses helps limit adverse effects and resistance pressure. Many conditions can be treated with shorter courses (5 to 7 days) rather than prolonged regimens.
12. Safety Concerns and Regulatory Actions
Over the past 15 years, regulatory bodies have released multiple safety warnings regarding fluoroquinolones. Key advisories:
- Tendon Rupture Warning (2008): Alerts prescribing clinicians to tendonitis and rupture, leading to black box warnings in the U.S.
- Peripheral Neuropathy (2013): The risk of potentially irreversible nerve damage.
- Neuropsychiatric Effects and Hypoglycemic Coma (2018): Additional FDA caution about mental health side effects and blood glucose disturbances.
- Aortic Aneurysm Risk (2018): A potential association with an increased risk of aortic aneurysm or dissection.
These warnings underscore the importance of appropriate patient selection and thorough risk assessment before prescribing fluoroquinolones.
13. Emerging Fluoroquinolones and Modifications
To overcome resistance and toxicity challenges, pharmaceutical research continues to explore:
- Structural Refinements
- Adjustments to the quinolone ring and side chains aim to widen antibacterial spectra, minimize resistance, and lower toxicities.
- Novel Delivery Systems
- Liposomal or nanoparticle formulations may achieve localized high concentrations with minimized side effects, proving valuable in complicated infections (e.g., inhaled formulations for cystic fibrosis).
- Combination Therapies
- Combining fluoroquinolones with β-lactams or other antimicrobial agents to achieve synergistic activity, especially in polymicrobial infections or MDR pathogens.
- Diagnostics Integration
- Rapid molecular diagnostics can identify resistance genes, assisting clinicians in rapid antibiotic selection and potentially limiting unnecessary fluoroquinolone usage.
14. Clinical Pearls and Best Practices
- Assess Renal Function
- Renal dose adjustments are often essential (levofloxacin, ciprofloxacin).
- Moxifloxacin is less dependent on renal clearance, making it an option in renal impairment (but not for UTIs due to limited urinary excretion).
- Monitor for Side Effects
- Counsel regarding assessment of musculoskeletal pain, tendon soreness, or mental changes.
- Watch for palpitations or changes on ECG if patient is at high risk for QT prolongation.
- Avoid in Certain Populations
- Growing caution in pediatric patients due to potential adverse musculoskeletal effects (though sometimes used for severe infections when benefits outweigh risks).
- Avoid or restrict in pregnant or lactating women, unless no safer alternative exists.
- Spacing with Cations
- Administer fluoroquinolones at least 2 hours before or after ingesting antacids, dairy products, or iron supplements to maintain absorption.
- Culture and Sensitivity
- Whenever possible, tailor antibiotic choice to the specific pathogen’s susceptibility profile—and consider local resistance patterns.
15. Future Directions
Although challenges like resistance and adverse events temper the once-universal usage of fluoroquinolones, these agents remain indispensable in modern therapy. Next-generation research focuses on:
- Developing new derivatives that retain potent activity while minimizing toxicity and circumventing existing resistance mechanisms.
- Optimizing stewardship programs, ensuring fluoroquinolones are used only when indicated and in appropriate doses.
- Investigating synergy with other treatment modalities (e.g., bacteriophage therapy, novel antimicrobial peptides) to extend the longevity of existing antibiotics.
Furthermore, as we refine pharmacogenomics and personalized medicine strategies, we may better predict which patients are at increased risk of severe fluoroquinolone-associated adverse reactions, thus improving patient safety.
16. Conclusion
Fluoroquinolones have reshaped the management of bacterial infections by providing broad-spectrum coverage, excellent oral bioavailability, and fast bactericidal action through the inhibition of DNA gyrase and topoisomerase IV. Their versatility spans respiratory infections, urinary tract infections, intra-abdominal infections, skin and soft tissue infections, and bone and joint infections, among others. Despite their success, side effects (like tendon rupture, QT interval prolongation, and CNS disturbances) and the rise of resistant strains call for cautious prescribing.
Key points to remember about fluoroquinolones:
- Mechanism: They target bacterial DNA synthesis by inhibiting topoisomerases.
- Pharmacokinetics: Favorable absorption and distribution allow convenient dosing and broad tissue coverage.
- Resistance: Emergence of resistant bacteria due to mutations in topoisomerase genes, efflux pumps, and plasmid-mediated mechanisms.
- Adverse Effects: Tendonitis, neuropathy, potential severe arrhythmias, and dysglycemia warrant prudent use.
- Stewardship: Emphasize tailoring therapy with culture data, limiting use to indicated cases, and reducing unnecessary exposure.
As healthcare systems adapt to global challenges of antibiotic resistance, fluoroquinolones must be reserved for optimal, evidence-based indications. Ongoing research, improved diagnostics, and stringent antibiotic stewardship will help preserve their efficacy for future generations.
References
- Goodman & Gilman’s The Pharmacological Basis of Therapeutics (13th Edition). Brunton LL, Hilal-Dandan R, Knollmann BC (Eds.). New York: McGraw Hill.
- Basic & Clinical Pharmacology (15th Edition). Katzung BG, Kruidering-Hall M, Trevor AJ (Eds.). New York: McGraw Hill.
- Rang & Dale’s Pharmacology (8th Edition). Rang HP, Dale MM, Flower RJ, Henderson G (Eds.). Elsevier.
- Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. Bennett JE, Dolin R, Blaser MJ (Eds.). Elsevier.
- Infectious Diseases Society of America (IDSA) Guidelines for the Treatment of Bacterial Infections and Antimicrobial Stewardship recommendations.
Medical Disclaimer
The medical information on this post is for general educational purposes only and is provided by Pharmacology Mentor. While we strive to keep content current and accurate, Pharmacology Mentor makes no representations or warranties, express or implied, regarding the completeness, accuracy, reliability, suitability, or availability of the post, the website, or any information, products, services, or related graphics for any purpose. This content is not a substitute for professional medical advice, diagnosis, or treatment; always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition and never disregard or delay seeking professional advice because of something you have read here. Reliance on any information provided is solely at your own risk.

