Introduction
Atropine—the prototypical naturally occurring belladonna alkaloid—has been used in medicine for well over a century. Extracted chiefly from Atropa belladonna (deadly night-shade), it remains indispensable in anesthesia, cardiology, ophthalmology, toxicology, and emergency medicine. Because it blocks muscarinic acetylcholine (ACh) receptors, atropine exemplifies the class of competitive antimuscarinic (parasympatholytic) agents.
1. Historical and Chemical Background
1.1 Botanical Source and Isolation
• Atropine is a tropane alkaloid derived from several Solanaceae species (Atropa belladonna, Datura stramonium, Hyoscyamus niger).
• It exists as a racemic mixture of dl-hyoscyamine; the l-isomer possesses virtually all antimuscarinic activity.
1.2 Structural Features
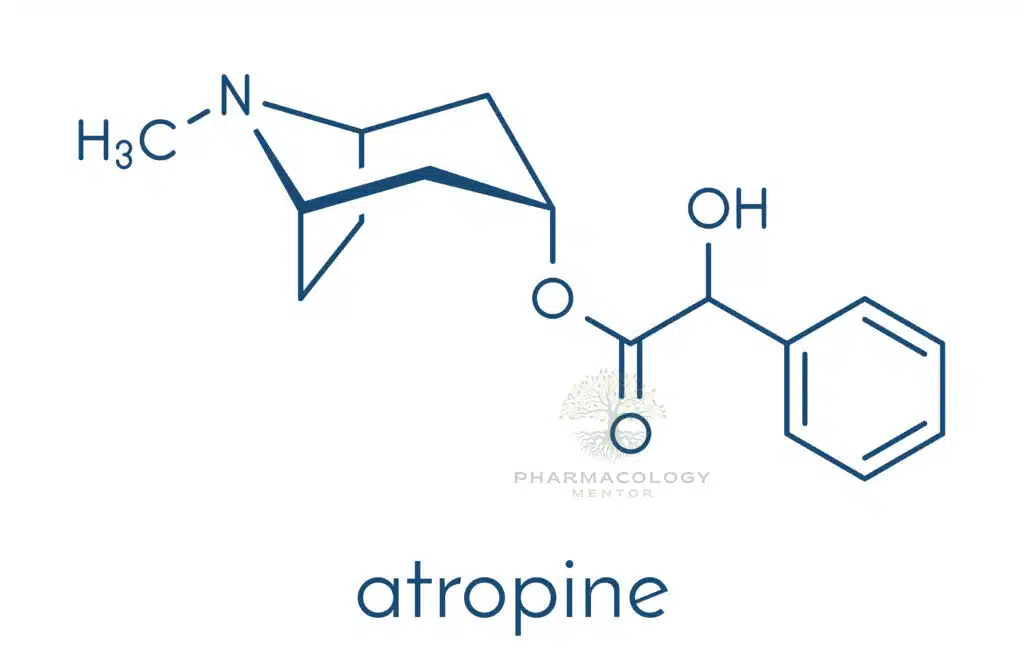
Atropine consists of a bicyclic tropane ring (tropine) esterified with tropic acid. Key structure–activity points:
• The cationic tertiary amine enables lipid solubility and central nervous system (CNS) penetration.
• Ester function is essential for high-affinity binding to muscarinic receptors; minor modifications can create semi-synthetic derivatives (e.g., homatropine, ipratropium).
• Because the molecule is non-polar at physiological pH, it crosses membranes easily, a property that distinguishes it from quaternary ammonium congeners (which have restricted CNS access).
2. Pharmacodynamics
2.1 Mechanism of Action
Atropine is a competitive, reversible antagonist at all five cloned muscarinic receptor subtypes (M₁–M₅), though tissue-specific selectivity exists (e.g., vagal SA-node M₂ vs. CNS M₁). By occupying the orthosteric ACh binding site, it prevents receptor activation, thereby inhibiting parasympathetic efferent activity and blocking muscarine-mediated reflexes.
2.2 Organs & Systems
• Heart (M₂): Vagal blockade → ↑ SA-node firing, ↑ AV-nodal conduction, modest ↑ contractility in atria. High doses may paradoxically induce tachyarrhythmias; low doses occasionally cause bradycardia via presynaptic M₁ blockade (disinhibition of ACh release).
• Vasculature: Little direct effect because most blood vessels have minimal parasympathetic tone; however, cutaneous vasodilation (“atropine flush”) may appear due to unknown mechanisms (histamine release, thermoregulatory reflexes).
• Eye (M₃): Relaxation of sphincter pupillae → mydriasis; paralysis of ciliary muscle → cycloplegia; ↓ lacrimation. Actions last 7–10 days after topical instillation.
• Respiratory: Bronchodilation and decreased bronchial secretions (M₃).
• Gastrointestinal & Biliary: Strong inhibition of salivary, gastric, duodenal, pancreatic secretions; decreased tone and motility of stomach and intestines (M₂/M₃). The LES tone may increase.
• Genitourinary: Relaxes detrusor (M₃) and slightly contracts trigone + internal sphincter → urinary retention in predisposed individuals.
• Sweat glands (sympathetic cholinergic): Inhibition → decreased thermoregulatory sweating, risk of hyperthermia, especially in children.
• CNS: Dose-related spectrum—mild stimulation and sedation at therapeutic doses; agitation, disorientation, hallucinations, and pronounced delirium at toxic concentrations. Atropine depresses vestibular reflexes (mechanism for anti-motion-sickness effect of related drugs like scopolamine).
2.3 Dose–Response Relationships
Typical dose ranges: 0.5 mg causes antisialagogue action; 1 mg generates moderate cardiac vagolysis; 2 mg yields pronounced tachycardia and mydriasis; >5 mg herald central toxicity (restlessness → coma). Lethal dose ~ 50 mg in adults, lower in infants.
3. Pharmacokinetics
3.1 Absorption
• Rapidly absorbed via oral, IM, or subcutaneous routes—peak plasma levels within 30–60 min.
• Ophthalmic formulations produce significant systemic levels in children; applying nasolacrimal occlusion mitigates absorption.
3.2 Distribution
• Widely distributed; volume of distribution ~ 2–4 L/kg.
• Crosses blood–brain and placental barriers; detectable in breast milk.
• 40–60 % plasma protein binding (α₁-acid glycoprotein).
3.3 Metabolism and Elimination
• Hepatic metabolism accounts for ~ 50 % (via CYP-mediated hydrolysis and N-demethylation).
• ~ 50 % excreted unchanged in urine; elimination half-life 2–3 h in adults, prolonged in neonates/elderly.
• Clearance is reduced in hepatic or renal impairment, but dose adjustment seldom critical except in extreme dysfunction.
4. Clinical Applications
4.1 Anesthesia and Surgery
• Premedication (antisialagogue): 0.3–0.6 mg IV/IM suppresses excessive airway secretions and vagally mediated bradycardia during instrumentation.
• Reversal of Non-depolarizing NMBAs: Combined with neostigmine (0.04–0.07 mg/kg) to offset muscarinic side effects of cholinesterase inhibitors. Glycopyrrolate often preferred because of minimal CNS penetration, but atropine remains standard in pediatric practice (rapid onset).
• Prevention/Treatment of oculo-cardiac reflex in ocular surgery.
4.2 Cardiology
• Symptomatic sinus bradycardia and AV-nodal block—first-line drug: 0.5–1 mg IV, repeat q3-5 min (max 3 mg).
• Historically for acute myocardial infarction-associated bradyarrhythmias; now used judiciously (tachycardia may worsen ischemia).
4.3 Ophthalmology
• Cycloplegic refraction in pediatrics.
• Treatment of uveitis/iritis to prevent synechiae and relieve ciliary spasm.
• Long-acting mydriasis useful for posterior segment examination; inconvenient for adults because of prolonged blurred vision.
4.4 Gastroenterology
• Antispasmodic in irritable-bowel, biliary, and renal colic (though newer agents often favored).
• Historically for peptic ulcer disease before PPIs/H₂ blockers.
4.5 Pulmonology
• Pre-bronchoscopy drying agent. Quaternary analogs (ipratropium, tiotropium) supersede atropine for chronic obstructive pulmonary disease (COPD) and asthma due to inhalational delivery and lower systemic effects.
4.6 Toxicology
• Antidote for organophosphorus (OP) and carbamate cholinesterase inhibitor poisoning. Dosing is titrated to drying of bronchial secretions and mitigation of bronchospasm rather than heart rate; massive doses (≥100 mg cumulative) may be required.
• Muscarinic mushroom poisoning (Clitocybe, Inocybe species).
• Cholinergic crisis from pyridostigmine or physostigmine overdose.
4.7 Others
• Termination of reflex-mediated muscarinic symptoms (e.g., mast cell–mediated bradycardia, carotid sinus hypersensitivity).
• Pre-radiology antisialogogue to improve head-and-neck imaging.
• Component of “DuoDote” auto-injector for military/first-responder OP exposure (Atropine + Pralidoxime).
5. Adverse Effects
Mnemonic “ABCDs” (Anorexia, Blurred vision, Constipation/Confusion, Dry mouth, Stasis of urine/Sweating decrease, Sedation):
• Ocular: Photophobia, blurred near vision, precipitate angle-closure glaucoma in narrow angles.
• Cardiac: Tachycardia, palpitations, potential ventricular arrhythmias in hyperthyroid or digitalized patients.
• Cutaneous: Dry, flushed skin; anhidrotic hyperthermia (“atropine fever” in children).
• CNS: Restlessness, irritability, hallucinations (“central anticholinergic syndrome”).
• GI/GU: Xerostomia, constipation, urinary retention, especially in benign prostatic hypertrophy (BPH).
• Respiratory: Viscid bronchial plugs in chronic lung disease.
• Misc: Rare idiosyncratic excitement in infants; decreased breast milk production.
6. Contraindications & Precautions
Absolute/relative:
• Narrow-angle (angle-closure) glaucoma or shallow anterior chamber.
• Prostatic hypertrophy with significant outflow obstruction.
• Paralytic ileus, pyloric stenosis, severe ulcerative colitis/megacolon.
• Tachyarrhythmias secondary to thyrotoxicosis or chronic heart failure.
• Myasthenia gravis (unless reversing cholinergic crisis).
• Hyperthermia (febrile children, hot environments).
• Pregnancy Category C: use only if benefits justify risk; crosses placenta but teratogenicity not proven.
• Geriatric: Highly sensitive to CNS delirium; use lower doses, avoid nighttime administration.
7. Drug Interactions
• Synergistic anticholinergicity with tricyclic antidepressants, antihistamines (1st gen), phenothiazines, MAO-Is, clozapine → severe dry mouth, tachycardia, hyperthermia.
• Opioids: Additive constipation + urinary retention; atropine mitigates vagal bradycardia.
• Digoxin: Concomitant use can exacerbate digoxin-induced arrhythmias (due to increased AV nodal conduction).
• Potassium chloride (solid oral forms): Reduced GI motility ↑ risk of ulceration.
• Cholinesterase inhibitors: Pharmacological antagonism (basis for toxicology antidotal pairing).
• Antacids & antidiarrheals containing adsorbents may reduce oral absorption of atropine.
8. Toxicology and Management
8.1 Clinical Picture of Overdose
“Dry as a bone, red as a beet, blind as a bat, hot as a hare, mad as a hatter, and full as a flask”:
• Mucosal dryness, hyperthermia, cutaneous vasodilation, mydriasis with cycloplegia, tachycardia, urinary retention, decreased bowel sounds, delirium → seizures, coma, respiratory failure.
8.2 Treatment
• Supportive: Cooling blankets, benzodiazepines for agitation/seizures, airway management.
• Antidote:Physostigmine 0.5–2 mg IV slowly; crosses BBB and reverses central as well as peripheral symptoms. Repeat q10–30 min PRN (watch for cholinergic signs).
• Enhanced elimination not useful (large Vd). Activated charcoal if presented early. Hemodialysis ineffective.
9. Special Populations
9.1 Pediatrics
• Higher vagal tone makes atropine particularly effective for bradycardia during laryngoscopy.
• Neonates have immature hepatic metabolism → prolonged half-life; dosing 0.02 mg/kg (minimum 0.1 mg to avoid paradoxical bradycardia).
• Susceptible to hyperthermia; caution in febrile states.
9.2 Geriatrics
• Enhanced sensitivity: confusion, urinary retention, glaucoma precipitation. Start at half adult dose.
9.3 Pregnancy and Lactation
• Crosses placenta; fetal tachycardia reported. Secreted in breast milk; may cause infantile constipation or anhidrosis—but serious toxicity rare.
9.4 Hepatic/Renal Dysfunction
• Moderate prolongation of half-life; single-dose therapy usually safe. Avoid chronic high-dose use without monitoring.
10. Emerging and Less Common Uses
• Pre-hospital combat care: auto-injector atropine/pralidoxime now standard; studies on intranasal or inhaled atropine ongoing.
• Cardiopulmonary resuscitation research: reevaluation of atropine’s role in PEA/asystole (AHA 2010 guidelines removed routine use, but some evidence hints benefit in certain subgroups).
• Central anticholinergic challenge test for diagnosing autonomic disorders (research tool).
• Checkpoint-inhibitor colitis: anecdotal use of anticholinergics to manage diarrhea.
• Optogenetic manipulations: atropine applied in neurophysiology experiments to block muscarinic modulation.
11. Comparative Pharmacology within the Antimuscarinic Class
| Feature | Atropine (tertiary) | Scopolamine (tertiary) | Glycopyrrolate (quaternary) | Ipratropium/Tiotropium (quaternary inhaled) |
| CNS penetration | Moderate | High (pronounced sedation) | Minimal | Minimal |
| Duration systemic | 2–4 h | 4–6 h | 2–4 h | Local 4–24 h |
| Primary clinical niche | Bradycardia, poisonings, ophthalmology | Motion sickness | Anesthesia antisialagogue | COPD/asthma |
12. Dose Forms and Administration Guidelines
• Parenteral (IV/IM/SC): 0.5 mg/mL, 1 mg/mL ampoules.
– Adult bradycardia: 0.5–1 mg IV (repeat to max 3 mg).
– OP poisoning: 2–6 mg IV/IM q5–10 min until secretions dry.
• Oral: 0.4 mg tablets; rarely used chronically today.
• Ophthalmic: 0.5 %, 1 % solution/ointment.
• Auto-injector: 2 mg per 0.7 mL; DuoDote or ATNAA.
• Nebulized: Not standard; experimental.Technique pearls:
• Always flush IV line pre- and post- dose when co-administered with cholinesterase inhibitors (incompatibility risk).
• Minimize accidental ocular exposure when handling OP antidote in the field (dark glasses for mydriasis).
• Apply pressure over lacrimal sac 1 min after ophthalmic drops to cut systemic absorption.
13. Laboratory/Monitoring Considerations
Routine serum atropine levels are not clinically used; instead, monitor:
• Heart rate/ECG for excessive tachycardia or Type II AV block reversal failure.
• Pupil size in OP poisoning (but treat to drying of secretions rather than pupil dilatation).
• Temperature in children.
• Urine output/bowel sounds for retention or paralytic ileus.
14. Molecular Pharmacology & Receptor Selectivity
• Muscarinic receptors are GPCRs: M₁, M₃, M₅ couple to Gq/11 → PLC activation; M₂, M₄ couple to Gi/o → ↓cAMP, open K⁺ channels.
• Crystallographic data show atropine’s bicyclic tropane forms hydrophobic contacts within TM3/6, while its cationic N interacts with Asp^3.32, and the ester carbonyl hydrogen-bonds to Asn^6.52.
• Atropine’s binding kinetics: on-rate 10⁷ M⁻¹ s⁻¹, off-rate 10⁻¹ s⁻¹ → K_d ~10 nM. Longer residence time at M₂ vs. M₃ explains durable vagolysis relative to GI effects.
15. Pharmacogenomics
Variants in CHRM2 (M₂) and CYP2D6 may modulate cardiac response and clearance, respectively, though clinical dose adjustment by genotype is not yet standard. Certain butyrylcholinesterase polymorphisms can alter baseline cholinergic tone, indirectly impacting atropine requirement during OP poisoning.
16. Future Directions
• Nanocarrier formulations aim to deliver atropine selectively to CNS in Alzheimer’s imaging studies.
• Selective M₂ allosteric antagonists under development may supersede atropine for bradycardia with fewer adverse effects.
• Integration into biosensor-controlled auto-injectors for real-time OP exposure treatment.
• Exploring atropine micro-dosing as prophylaxis for excessive vagal reflexes during ablative cardiac procedures.
17. Summary
Atropine remains the gold-standard antimuscarinic, prized for its versatility, predictable kinetics, and established therapeutic indices. From reversing deadly cholinergic crises to preventing intraoperative bradycardia and facilitating ocular examinations, its clinical footprint spans virtually every medical specialty. Familiarity with atropine’s mechanism, dosing strategies, and toxicity management is therefore essential for any clinician.
Select Bibliography
- Brunton LL, Hilal-Dandan R, Knollmann BC (eds). Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 14th ed. New York: McGraw-Hill; 2023.
- Katzung BG, Vanderah TW (eds). Basic & Clinical Pharmacology, 15th ed. New York: McGraw-Hill; 2021.
- Rang HP, Dale MM, Ritter JM, Flower RJ, Henderson G. Rang & Dale’s Pharmacology, 10th ed. London: Elsevier; 2023.
- Neal MJ. Medical Pharmacology at a Glance, 9th ed. Oxford: Wiley-Blackwell; 2020.
- Tripathi KD. Essentials of Medical Pharmacology, 9th ed. New Delhi: Jaypee; 2019.
- Butterworth JF, Mackey DC, Wasnick JD. Morgan & Mikhail’s Clinical Anesthesiology, 7th ed. New York: McGraw-Hill; 2022.
- Mills S, Hillhouse B. Dictionary of Cardiac Antidotes. London: Springer; 2022.
- Barash PG, et al. Clinical Anesthesia, 9th ed. Philadelphia: Wolters Kluwer; 2022.
- Lippincott Illustrated Reviews: Pharmacology, 8th ed. Philadelphia: Wolters Kluwer; 2021.
- Stevens CW, Brenner GM. Pharmacology, 6th ed. Philadelphia: Elsevier; 2023.









