Scope: Pathophysiology, Drug Classifications, Mechanisms of Action, Clinical Pharmacology, and Therapeutic Guidelines.
I. Introduction and Pathophysiology
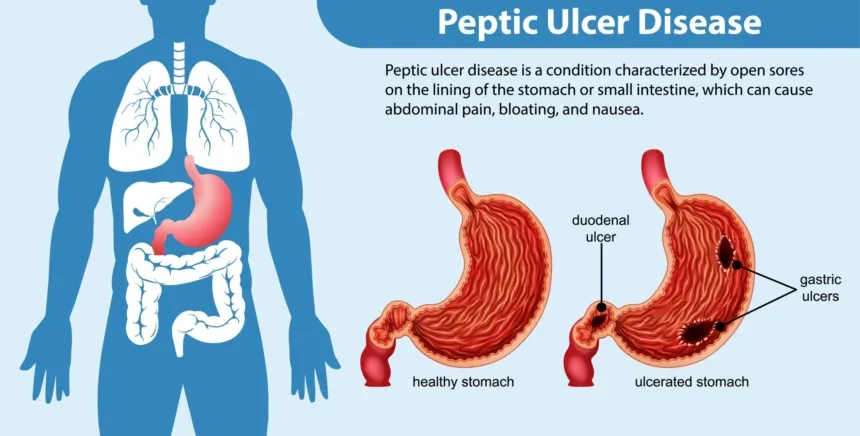
Peptic ulcer disease (PUD) is a chronic, relapsing inflammatory disorder characterized by a breach in the mucosa of the stomach (gastric ulcer) or the proximal duodenum (duodenal ulcer) extending through the muscularis mucosae. The pathophysiology of PUD is best understood as a disruption of the delicate equilibrium between aggressive factors and defensive mechanisms.
The Balance Hypothesis
A peptic ulcer develops when the aggressive factors overwhelm the mucosal defenses. Pharmacotherapy aims to restore this balance by either reducing aggression or bolstering defense.
| Aggressive Factors | Defensive Factors |
|---|---|
| Gastric Acid (HCl): Direct corrosion. | Mucus Layer: Physical barrier against acid/pepsin. |
| Pepsin: Proteolytic enzyme. | Bicarbonate (HCO3–): Neutralizes acid at the epithelial surface. |
| Helicobacter pylori: Bacterial infection. | Prostaglandins (PGE2, PGI2): Stimulate mucus/bicarbonate; maintain blood flow. |
| NSAIDs: Inhibit protective prostaglandin synthesis. | Mucosal Blood Flow: Removes acid; supplies oxygen/nutrients for repair. |
Regulation of Acid Secretion
The central target of most anti-ulcer drugs is the parietal cell. The final common pathway for acid secretion is the Proton Pump (H+/K+-ATPase).
The parietal cell is stimulated by three primary secretagogues acting on specific basolateral receptors:
- Histamine: Binds to H2 receptors (Gs-coupled) → increases cAMP → activates Protein Kinase A.
- Acetylcholine: Binds to M3 muscarinic receptors (Gq-coupled) → increases intracellular Ca2+.
- Gastrin: Binds to CCK2 receptors (Gq-coupled) → increases intracellular Ca2+.
II. Agents Reducing Intragastric Acidity
A. Proton Pump Inhibitors (PPIs)
Agents: Omeprazole, Esomeprazole, Lansoprazole, Pantoprazole, Rabeprazole.
1. Mechanism of Action
PPIs are prodrugs. They are weak bases that circulate in the blood in an inactive form.
- Ion Trapping: They diffuse into the highly acidic secretory canaliculi (pH < 1.0).
- Activation: In this environment, the PPI is protonated and forms a reactive sulfenamide cation.
- Irreversible Inhibition: This forms a covalent disulfide bond with cysteine residues (specifically Cys813) on the H+/K+-ATPase pump.
2. Clinical Uses & Pharmacokinetics
- Uses: PUD, GERD, Zollinger-Ellison Syndrome, NSAID prophylaxis, H. pylori eradication.
- Metabolism: Hepatic via CYP2C19 and CYP3A4. Note: Genetic polymorphism in CYP2C19 (common in Asian populations) can affect efficacy.
3. Adverse Effects & Interactions
- Nutritional Deficiencies (B12, Iron, Calcium).
- Increased risk of bone fractures and C. difficile infection.
- Rebound hypersecretion upon stopping.
B. Potassium-Competitive Acid Blockers (P-CABs)
Agent: Vonoprazan.
This is a newer class of drugs. Unlike PPIs, P-CABs compete reversibly with K+ ions at the pump. They do not require acid activation. They offer a rapid onset (day 1) and are highly effective in H. pylori eradication.
C. H2 Receptor Antagonists (H2RAs)
Agents: Famotidine, Nizatidine, Cimetidine.
- Mechanism: Reversible block of H2 receptors. Highly effective for nocturnal acid secretion.
- Adverse Effects (Cimetidine): Cimetidine is a potent CYP inhibitor and has anti-androgenic effects (gynecomastia) in men.
- Tolerance: Rapid tolerance (tachyphylaxis) develops within 3 days, limiting long-term use.
III. Agents Neutralizing Acid (Antacids)
Antacids are weak bases that react with gastric hydrochloric acid to form a salt and water.
| Type | Agents | Features | Adverse Effects |
|---|---|---|---|
| Systemic | Sodium Bicarbonate | Rapid onset; absorbed. | Metabolic alkalosis; Fluid retention. |
| Non-Systemic | Magnesium Hydroxide | Potent; poor absorption. | Diarrhea (osmotic). |
| Aluminum Hydroxide | Slow acting. | Constipation; Hypophosphatemia. | |
| Calcium Carbonate | Potent; rapid. | Rebound acid; Kidney stones. |
IV. Mucosal Protective Agents
A. Sucralfate
Forms a viscous, sticky polymer in acid (pH < 4) that adheres to the ulcer crater ("Band-Aid" effect). Note: Requires acid to work; do not give with PPIs.
B. Misoprostol (Prostaglandin E1 Analogue)
Stimulates mucus/bicarbonate secretion and inhibits acid. Specifically indicated for prevention of NSAID-induced ulcers.
C. Bismuth Compounds
Coats the ulcer and possesses direct antimicrobial activity against H. pylori. Causes harmless blackening of stool/tongue.
V. Pharmacotherapy of H. pylori (2024 Update)
The goal is bacterial eradication. High intragastric pH is required to optimize antibiotic efficacy.
1. First-Line: Bismuth Quadruple Therapy (BQT)
Preferred due to rising clarithromycin resistance.
- PPI (b.i.d.)
- Bismuth Subcitrate (q.i.d.)
- Tetracycline (500 mg q.i.d.)
- Metronidazole (q.i.d.)
- Duration: 10–14 days.
2. The “Modern” Approach: Vonoprazan-Based Therapy
Superior acid suppression leads to higher eradication rates.
- Dual Therapy: Vonoprazan + Amoxicillin.
- Triple Therapy: Vonoprazan + Amoxicillin + Clarithromycin.
3. Clarithromycin Triple Therapy (Restricted)
Only use if local resistance is known to be < 15%. (PPI + Clarithromycin + Amoxicillin).
VI. Summary of Drug Classes
| Drug Class | Prototype | Mechanism Target | Main Limitation |
|---|---|---|---|
| PPIs | Omeprazole | Irreversible H+/K+ block | Bone fracture risk; C. diff risk. |
| P-CABs | Vonoprazan | Reversible K+ competition | Newer agent; cost. |
| H2 Blockers | Famotidine | Histamine H2 block | Tachyphylaxis (tolerance). |
| Prostaglandins | Misoprostol | EP3 agonist | Diarrhea; Abortifacient. |
| Coating Agents | Sucralfate | Physical barrier | Drug binding interactions. |
VII. Conclusion
The pharmacotherapy of peptic ulcer disease relies on restoring the balance between aggressive and defensive factors. While PPIs remain the standard for acid suppression, the management of H. pylori is shifting toward Bismuth Quadruple Therapy and Vonoprazan-based regimens to combat antibiotic resistance.
📚 AI Pharma Quiz Generator
🎉 Quiz Results
Medical Disclaimer
The medical information on this post is for general educational purposes only and is provided by Pharmacology Mentor. While we strive to keep content current and accurate, Pharmacology Mentor makes no representations or warranties, express or implied, regarding the completeness, accuracy, reliability, suitability, or availability of the post, the website, or any information, products, services, or related graphics for any purpose. This content is not a substitute for professional medical advice, diagnosis, or treatment; always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition and never disregard or delay seeking professional advice because of something you have read here. Reliance on any information provided is solely at your own risk.