Procainamide is a Class IA antiarrhythmic that blocks fast INaINa channels and prolongs repolarization via its active metabolite N-acetylprocainamide (NAPA), making it useful for acute management of a variety of ventricular and supraventricular tachyarrhythmias when carefully dosed and monitored.
Classification and overview
Procainamide belongs to the Vaughan Williams Class IA group (with quinidine and disopyramide), characterized by intermediate kinetics of sodium-channel block that slows phase‑0 upstroke, decreases conduction velocity, and prolongs action potential duration and effective refractory period.
Unlike quinidine, procainamide’s major metabolite NAPA contributes prominent class III effects (prolonged repolarization) without additional sodium-channel block, which has important implications for QT prolongation and torsades risk.
Mechanism of action
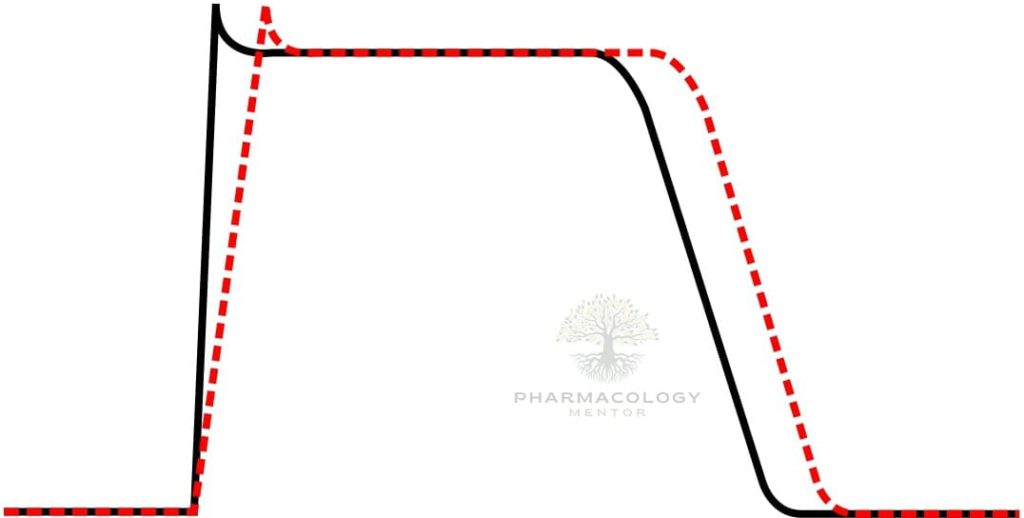
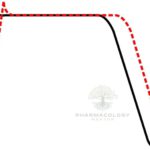
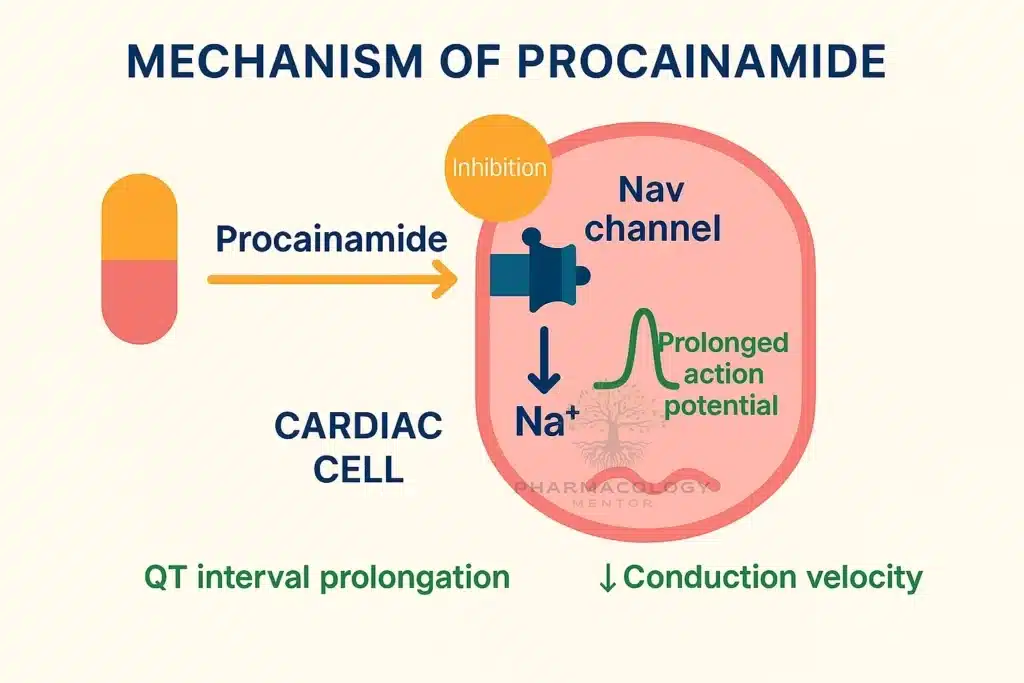
Procainamide causes use‑ and voltage‑dependent block of cardiac fast INaINa channels, widening QRS by slowing His–Purkinje and ventricular conduction, and it modestly blocks outward K+K+ currents through its metabolite NAPA, which prolongs QT by lengthening ventricular repolarization.
On ECG, procainamide typically prolongs PR (AV nodal conduction), widens QRS (intraventricular conduction), and—via NAPA—prolongs QTc, patterns that guide safety monitoring during infusion.
Pharmacokinetics and metabolism
Procainamide is variably acetylated hepatically by N‑acetyltransferase to NAPA, with genetically determined acetylator status and renal function strongly influencing PA:NAPA ratios and overall exposure.
Both procainamide and NAPA are renally cleared, so renal impairment increases accumulation—particularly of NAPA—and heightens risks of QT prolongation and torsades, necessitating dose adjustment and therapeutic drug monitoring.
Therapeutic drug monitoring
Typical therapeutic serum ranges are procainamide 4.0–10.0 mcg/mL and NAPA 12.0–18.0 mcg/mL, with toxicity likely if procainamide exceeds 12 mcg/mL or NAPA exceeds 40 mcg/mL; combined “active” concentrations are often interpreted together in practice.
Slow acetylators (higher PA:NAPA) have greater risk of lupus‑like autoimmunity, while rapid acetylators and renal impairment predispose to higher NAPA levels and QT‑mediated proarrhythmia, supporting individualized targets and interval monitoring.
Indications (acute care focus)
Procainamide treats hemodynamically stable monomorphic wide‑QRS tachycardia (likely VT) with recommended controlled IV infusion, and it is an option for supraventricular tachyarrhythmias including AF with pre‑excitation when AV‑nodal blockers are undesirable.
As a sodium-channel blocker without beta‑blocking or calcium‑channel effects, procainamide may also be used diagnostically to unmask Brugada ECG patterns during controlled challenge in specialized settings.
Dosing and administration (ACLS‑aligned)
For stable wide‑QRS tachycardia, infuse 20–50 mg/min until the arrhythmia terminates, hypotension occurs, QRS widens by more than 50%, or a maximum dose of 17 mg/kg is reached; follow with maintenance 1–4 mg/min.
Avoid procainamide in patients with prolonged QT or decompensated heart failure per tachycardia algorithms, and stop the infusion promptly at defined endpoints (hypotension, QRS>50%, arrhythmia suppression, or dose limit).
Electrophysiology–clinical correlations
Acute QRS widening during infusion reflects progressive sodium-channel block and is a signal to slow or stop the infusion; progressive QT prolongation during or after loading suggests NAPA accumulation and torsades risk, especially with renal impairment.
NAPA coadministration experimentally prolongs procainamide’s elimination half‑life and potentiates QTc prolongation, explaining clinical scenarios in which prolonged QT occurs despite “therapeutic” procainamide levels.
Adverse effects and toxicity
The principal acute risk is hypotension with rapid infusion due to negative inotropy and vasodilation, which is minimized by controlled rates and continuous blood pressure and ECG monitoring.
Torsades de pointes can occur from repolarization prolongation—often driven by NAPA—particularly with renal failure, preexisting long QT, electrolyte abnormalities, or concurrent QT‑prolonging drugs.
Chronic or subacute use can cause a lupus‑like syndrome with arthralgias, serositis, fever, and ANA positivity, more common in slow acetylators, and it typically resolves after discontinuation.
Hematologic toxicity including neutropenia and agranulocytosis is rare but serious, warranting periodic blood counts during extended therapy or when clinically indicated.
Contraindications and cautions
Avoid procainamide in torsades or baseline QT prolongation, in decompensated heart failure during tachycardia management, and in high‑grade AV block without pacing support due to risks of proarrhythmia and hemodynamic compromise.
Use particular caution in renal impairment, slow acetylators (autoimmunity), and in combination with other QT‑prolonging agents; consider alternatives if significant hypotension develops during loading.
Drug interactions and special issues
Histamine H2‑receptor antagonists such as cimetidine and ranitidine reduce renal clearance of procainamide and NAPA, raising levels and increasing toxicity risk; dose and monitoring should be adjusted accordingly.
Combined exposure to other QT‑prolonging antiarrhythmics or electrolyte depletion amplifies torsades risk, underscoring correction of potassium and magnesium and careful selection of adjuncts.
Monitoring checklist
- Continuous ECG for PR, QRS, and QTc dynamics during infusion and early maintenance, with stop criteria for hypotension, QRS >50% from baseline, or arrhythmia suppression.
- Serum procainamide and NAPA concentrations to guide dosing and investigate toxicity, with renal function–guided intervals; CBC and ANA when prolonged courses or symptoms raise concern.
Use in specific scenarios
In pre‑excited AF (e.g., WPW), procainamide can slow accessory pathway conduction and organize rhythm without preferential AV‑nodal block, making it a useful acute option where nodal blockers may be hazardous.
As a pharmacologic alternative to immediate cardioversion in stable monomorphic VT, procainamide has favorable termination data in selected patients when administered under close hemodynamic and ECG surveillance.
Quick reference table
High‑yield MBBS points
Class IA drugs slow conduction and prolong repolarization; procainamide is distinguished by an active metabolite (NAPA) that behaves as a class III agent and drives QT‑related risks.
ACLS‑consistent dosing uses slow, controlled infusion with strict stop criteria and continuous ECG/BP monitoring, avoiding use in prolonged QT or acute heart failure.
Therapeutic drug monitoring of both procainamide and NAPA is essential in renal dysfunction or unexplained QT/QRS changes, and vigilance for lupus‑like features and blood dyscrasias is required during ongoing therapy.
References
- Ritter JM, Flower R, Henderson G, et al. Rang & Dale’s Pharmacology. 10th ed. London: Elsevier; 2024. Class I antiarrhythmics and electrophysiology.
- Pritchard B, Thompson H. Procainamide. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2025.
- Funck‑Brentano C, et al. Pharmacokinetic and pharmacodynamic interaction of N‑acetylprocainamide and procainamide. Clin Pharmacol Ther. 1989;45(6):668‑77.
- American Heart Association. Adult Tachycardia With a Pulse Algorithm (2020).
- Mayo Clinic Laboratories. Procainamide and N‑Acetylprocainamide, Serum: Reference Values and Interpretation. 2025.
- Ashida K, et al. Long QT syndrome caused by N‑acetylprocainamide in a hemodialysis patient. J Cardiol Cases. 2015;11(4):137–40.
- WHO/INCHEM. Procainamide (PIM 436): adverse reactions, lupus‑like syndrome, and agranulocytosis.
📚 AI Pharma Quiz Generator
🎉 Quiz Results
Medical Disclaimer
The medical information on this post is for general educational purposes only and is provided by Pharmacology Mentor. While we strive to keep content current and accurate, Pharmacology Mentor makes no representations or warranties, express or implied, regarding the completeness, accuracy, reliability, suitability, or availability of the post, the website, or any information, products, services, or related graphics for any purpose. This content is not a substitute for professional medical advice, diagnosis, or treatment; always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition and never disregard or delay seeking professional advice because of something you have read here. Reliance on any information provided is solely at your own risk.