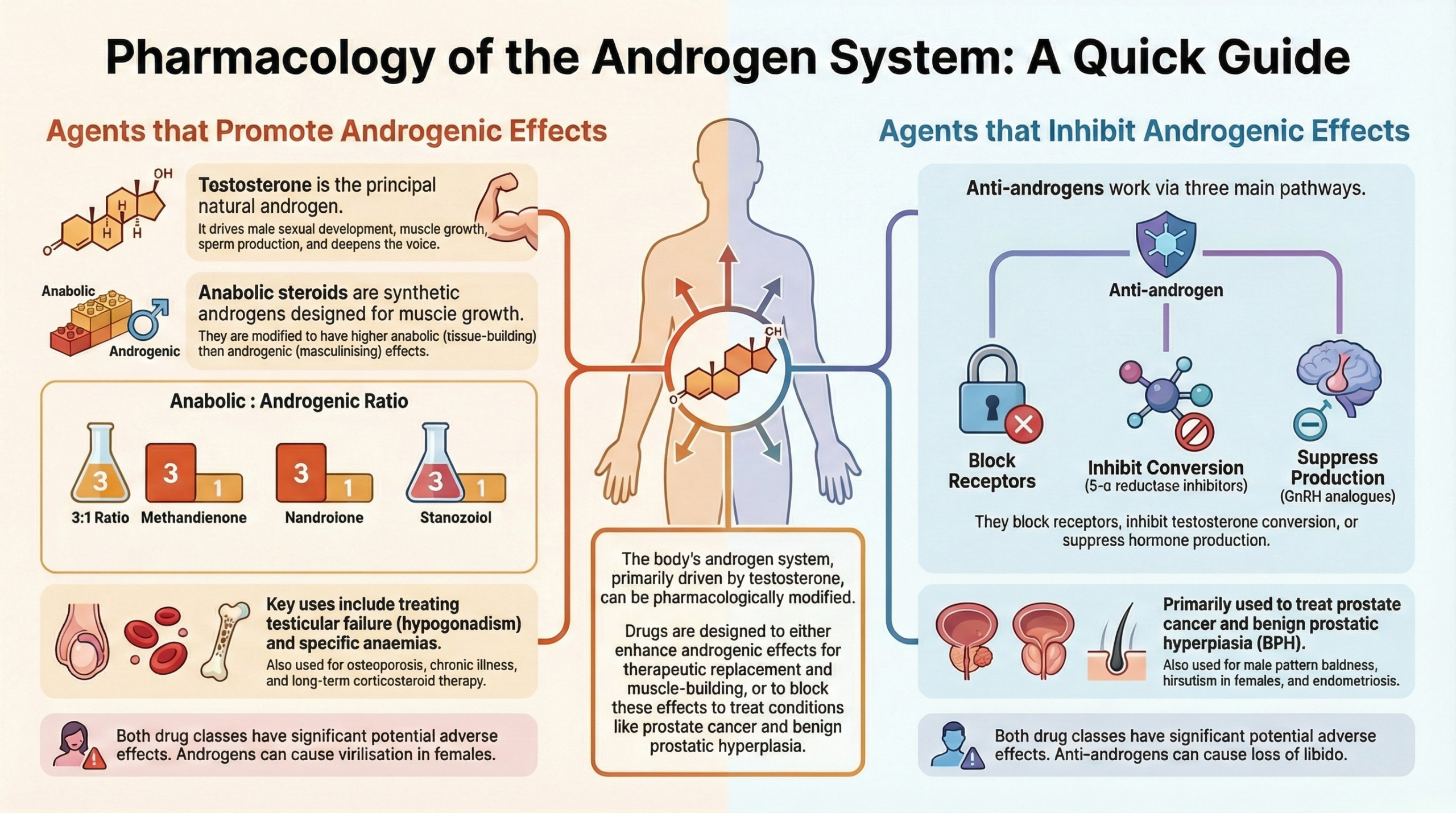
1. Introduction: Beyond the “Male Hormone”
Androgens are a class of steroid hormones often simplified as “male hormones,” but their physiological reach extends far beyond that label, playing crucial roles in the development and health of both sexes. These substances are responsible for the development of male secondary sexual characteristics and have profound effects on muscle mass, bone density, red blood cell production, and even behavior.
This article provides a comprehensive overview of the pharmacology surrounding these powerful molecules. We will explore the fundamental physiology of natural androgens, the therapeutic applications and risks of synthetic preparations, and the mechanisms of anabolic steroids. Furthermore, we will discuss into the drugs designed to counteract their effects—anti-androgens—and those that modulate the hormonal axis controlling their production, such as GnRH modulators. Finally, we will touch upon the emerging, investigational class of Selective Androgen Receptor Modulators (SARMs) to provide a complete picture of this complex pharmacological landscape.
2. The Foundation: Understanding Androgen Physiology
2.1. Defining Androgens
Androgens are substances, both natural and synthetic, that induce the development of male secondary sexual characteristics. It is a generic term for any steroidal drug with masculinizing properties. The primary natural androgens in the body are:
- Testosterone
- Dihydrotestosterone (DHT)
- Androstenedione
Testosterone is the principal androgen and serves as a prohormone, meaning it can be converted into other active hormones. Specifically, it can be converted into the more potent androgen, DHT, or into the primary female sex hormone, Oestradiol.
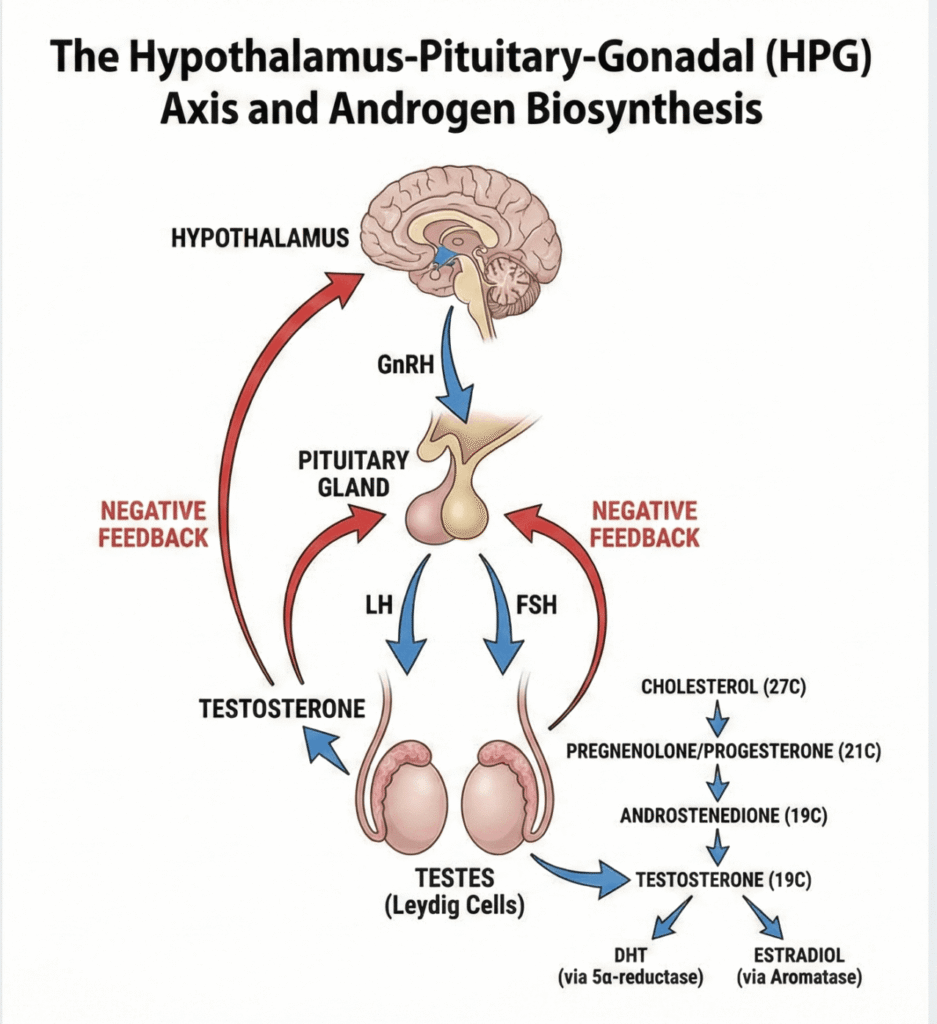
2.2. The Building Blocks: Steroid Chemistry and Biosynthesis
All steroid hormones, including androgens, are derived from a common chemical structure known as the Cyclopentanoperhydrophenanthrene nucleus. The biosynthesis pathway begins with cholesterol and proceeds through a series of enzymatic conversions that reduce the number of carbon atoms:
- Cholesterol (27 carbons) is the starting precursor.
- It is converted into Pregnane derivatives (21 carbons), which include progesterone and corticoids.
- These are then converted into Androstane derivatives (19 carbons), which are the androgens.
- Finally, androgens can be aromatized into Estrane derivatives (18 carbons), the estrogens.
The primary site of natural androgen synthesis is the testes, where Leydig cells produce Testosterone. Insignificant amounts are also produced by the adrenal glands, and small quantities are synthesized by the ovaries in women. Androsterone, a metabolite with approximately one-tenth the activity of testosterone, is excreted in the urine.
2.3. Regulation of Androgen Secretion: The HPG Axis
Androgen production is tightly regulated by a hormonal feedback system known as the Hypothalamus-Pituitary-Gonadal (HPG) axis. This cascade works as follows:
- The Hypothalamus releases Gonadotropin-Releasing Hormone (GnRH).
- GnRH stimulates the Pituitary gland to release Luteinizing Hormone (LH) and Follicle-Stimulating Hormone (FSH).
- LH travels to the testes and stimulates the Leydig cells to produce Testosterone.
- Testosterone then exerts a negative feedback effect, signaling the hypothalamus and pituitary to decrease their release of GnRH and LH, thereby maintaining hormonal balance.
2.4. Mechanism of Action at the Cellular Level
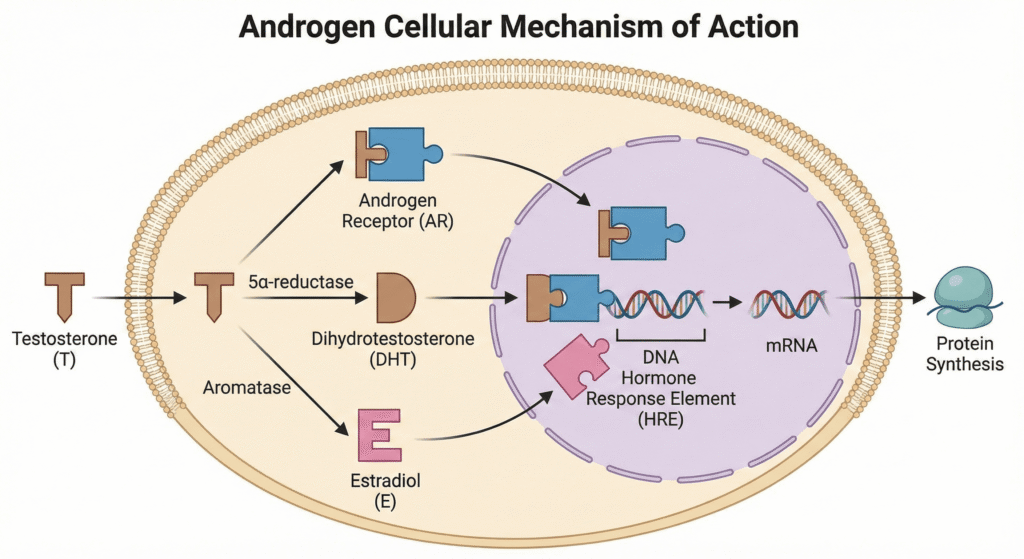
Once Testosterone enters a target cell, it can follow one of three pathways to exert its effects:
- It can bind directly to an androgen receptor (T-rec).
- It can be converted to Dihydrotestosterone (DHT) by the enzyme 5-α-reductase. DHT, a more potent androgen, then binds to an androgen receptor (DHT-rec).
- It can be converted to Estradiol (E) by the enzyme Aromatase. Estradiol then binds to an estrogen receptor (E-rec).
After binding, these hormone-receptor complexes move into the cell nucleus. There, they bind to specific DNA sequences, regulating the transcription of messenger RNA (mRNA). This process ultimately alters protein synthesis, leading to the wide-ranging physiological effects of androgens and estrogens.
3. The Pharmacology of Therapeutic Androgens
3.1. Physiological Actions of Testosterone and DHT
Testosterone and its potent metabolite DHT are responsible for a diverse range of physiological actions throughout the body:
- Growth of genitals in boys.
- Development of facial, pubic, and axillary hair.
- Increased muscular development.
- Growth of the larynx, leading to a deepening of the voice.
- Promotes closure of epiphyseal plates, which stops longitudinal bone growth at the end of puberty.
- Thickening of the skin and loss of subcutaneous fat.
- Behavioral changes associated with masculinity.
- Stimulation of sperm production (spermatogenesis).
- At larger, supraphysiological doses, can cause testicular atrophy due to HPG axis suppression.
- A nitrogen-retaining (anabolic) effect, promoting protein synthesis.
- Increased secretion of Erythropoietin, leading to higher red blood cell production.
- Adverse changes in lipid profiles, including increased Low-Density Lipoprotein (LDL) and decreased High-Density Lipoprotein (HDL).
3.2. Pharmacokinetics (ADME)
- Absorption: When taken orally, natural testosterone undergoes high first-pass metabolism in the liver, rendering it ineffective. For this reason, therapeutic administration typically relies on intramuscular injections or synthetic preparations designed to bypass this issue.
- Distribution: Testosterone is highly protein-bound in the bloodstream (98%), primarily to Sex Hormone-Binding Globulin (SHBG) and albumin.
- Metabolism: It is metabolized by liver enzymes into weaker metabolites like Androsterone and Etiocholanolone. A small quantity is also converted into Oestradiol via aromatization.
- Excretion: The metabolites are conjugated (made water-soluble) in the liver and excreted in the urine.
3.3. Androgen Preparations and Formulations
A variety of formulations are available to deliver therapeutic androgens effectively.
Injectable Testosterone Esters: Testosterone is esterified (e.g., as enanthate, cypionate, propionate, undecanoate) and formulated in oil for deep intramuscular injection. This slows its release, making it effective for weeks to months. However, this method can cause wide fluctuations in serum testosterone concentrations, which may lead to undesirable fluctuations in energy, mood, and libido. Dosing intervals vary, with enanthate/cypionate typically administered every 1-2 weeks and the long-acting undecanoate ester every 10 weeks.
Aqueous Testosterone Suspension: This injectable formulation consists of unesterified testosterone in a water-based solution. It has a much faster onset and shorter duration of action compared to oil-based esters, typically dosed at 50-100 mg every 2-3 days, but is less commonly used today.
Transdermal Preparations: FDA-approved transdermal patches and gels are available for treating male hypogonadism. Gels are applied once daily and provide relatively steady serum testosterone concentrations. Patches are typically applied to the back, abdomen, or thigh.
Orally Active Androgens: Several orally active synthetic androgens have been developed, including Methyltestosterone, Fluoxymesterone, and Mesterolone. Testosterone undecanoate is also available as an oily solution that is absorbed through the lymphatic system, bypassing the liver’s first-pass metabolism. These preparations generally have submaximal androgenic efficacy. Critically, 17α-alkylated androgens like Methyltestosterone and Fluoxymesterone carry a significant risk of causing cholestatic jaundice and hepatotoxicity.
Subcutaneous Implants: Pellets of crystalline testosterone can be implanted subcutaneously, typically in the abdominal wall or thigh. This formulation provides a slow, sustained release of the hormone over several months, offering stable blood levels but requiring a minor surgical procedure for insertion and removal.
4. Clinical Applications and Risks of Androgen Therapy
4.1. Therapeutic Uses of Androgens
Androgen therapy is indicated for a range of medical conditions:
- Androgen replacement: For testicular failure, including primary and secondary hypogonadism.
- Senile Osteoporosis: To improve bone density.
- Refractory Anemia: To stimulate red blood cell production via increased erythropoietin secretion.
- Carcinoma of the breast: Specific agents like Testolactone are used. As a progesterone derivative and aromatase inhibitor, it works by preventing the conversion of androgens to estrogens that could fuel hormone-sensitive tumors.
- Hereditary angioneurotic edema.
- To counteract catabolic states: Androgens are used to promote a positive nitrogen balance and prevent muscle wasting in conditions such as severe burns, chronic illness, and during long-term corticosteroid therapy.
- Hypopituitarism and Ageing: For replacement purposes.
4.2. Adverse Effects
The use of androgens is associated with significant adverse effects, which differ based on sex and age.
In Females:
- Virilizing effects, including masculinization, acne, and hirsutism (excess hair growth).
- Frontal baldness (male-pattern hair loss).
- Shrunken breasts.
- Deepening of the voice.
In Males and General:
- Feminizing side effects such as gynecomastia (breast development), particularly in children.
- In children: Precocious puberty and stunted growth due to premature closure of the bone growth plates.
- Enlargement of the prostate.
- Atherosclerosis due to worsened lipid profiles (higher LDL, lower HDL).
- Oedema (fluid retention).
- Acne.
- Frequent, sustained, and often painful erections (priapism).
- Hepatotoxicity: Cholestatic jaundice (particularly with fluoxymesterone, oxymetholone, and stanozolol) and a risk of hepatic carcinoma with long-term use of agents like methyltestosterone are serious concerns with 17α-alkylated androgens.
4.3. Contraindications
Androgen therapy is strictly contraindicated in the following conditions:
- Carcinoma of the prostate
- Carcinoma of the male breast
- Significant liver or kidney disease
- During pregnancy
5. Anabolic Steroids: Building Mass, Managing Risk
5.1. Definition and Rationale
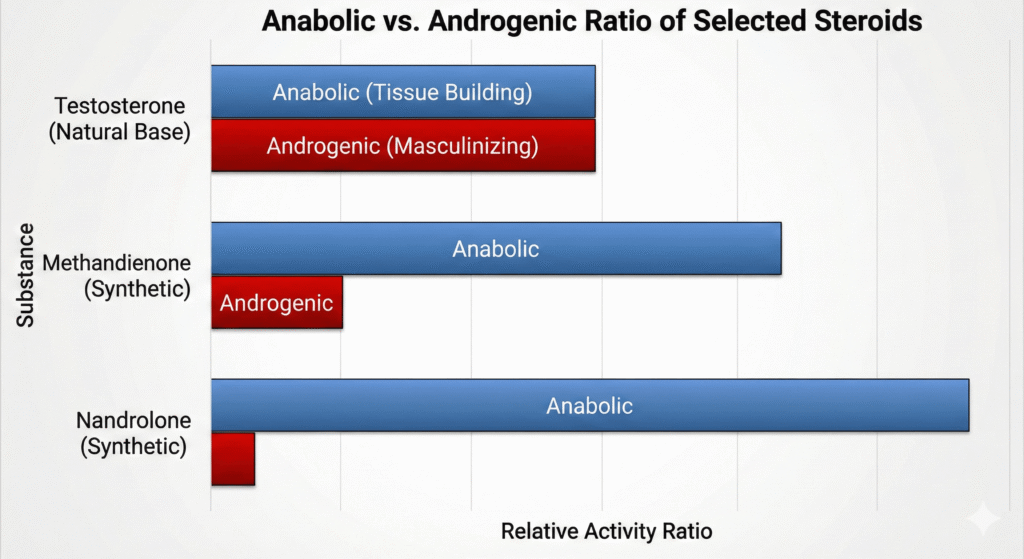
Anabolic steroids are synthetic androgens that have been chemically modified to enhance their anabolic (tissue-building) properties while reducing their androgenic (masculinizing) effects. The primary examples include Nandrolone, Oxymetholone, Stanozolol, and Methandienone.
5.2. Key Agents and Properties
These drugs are often characterized by their anabolic-to-androgenic activity ratio.
| Drug | Anabolic:Androgenic Ratio | Preparation & Dose |
| Methandienone | 3:1 | 5–15 mg/day, p.o. |
| Nandrolone phenylpropionate | 3:1 | 10–50 mg/wk, i.m. |
| Nandrolone decanoate | 3:1 | 25–50 mg/3 wk, i.m. |
| Stanozolol | 3:1 | 2-6 mg/day, p.o. |
5.3. Uses and Side Effects
Clinical applications of anabolic steroids include:
- Osteoporosis in elderly males (though bisphosphonates are now preferred).
- Catabolic states with a negative nitrogen balance.
- Refractory anemias: Specifically for hypoplastic, hemolytic, and malignancy-associated anemias.
- Short stature (a controversial use).
- They are also widely used illicitly to enhance athletic performance.
Anabolic steroids share the same side effect profile as other androgens. Specifically, Oxymetholone and Stanozolol are known to produce jaundice and worsen the lipid profile.
6. Blocking the Signal: A Guide to Anti-Androgens
Anti-androgens are drugs that counteract the effects of androgens through various mechanisms.
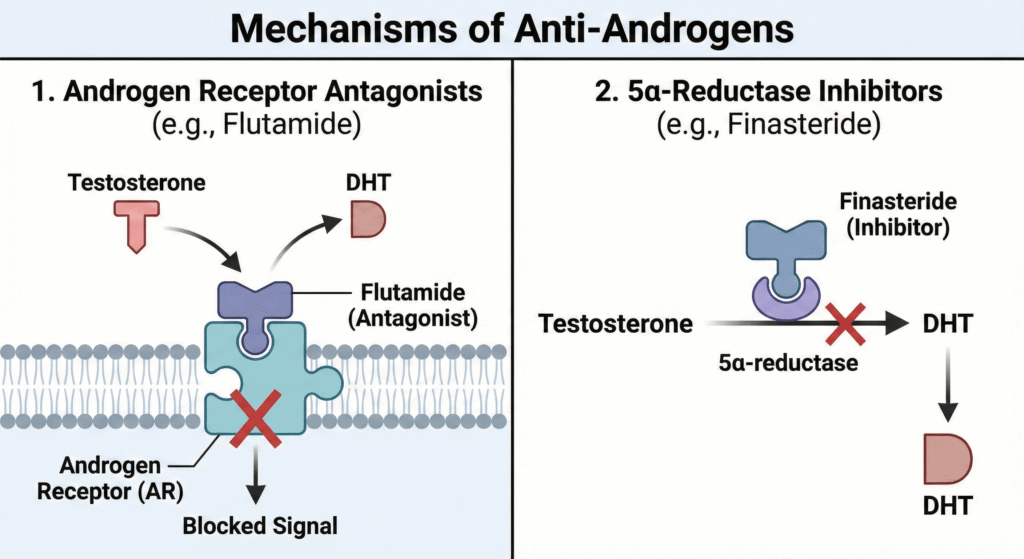
6.1. Androgen Receptor Antagonists
These drugs work by directly blocking the androgen receptor, preventing testosterone and DHT from binding.
- Cyproterone Acetate: A weak androgen receptor blocker that also decreases gonadotropin secretion. It is used to treat acne, male pattern baldness, hirsutism, carcinoma of the prostate, and precocious puberty.
- Flutamide & Bicalutamide: Flutamide is a non-steroidal antagonist used to treat prostate cancer (typically in combination with a GnRH agonist) and female hirsutism; however, it is associated with hepatotoxicity. Bicalutamide is a more potent, longer-acting, and less hepatotoxic alternative also used for metastatic prostate cancer.
6.2. 5-α-Reductase Inhibitors
This class of drugs inhibits the 5-α-reductase enzyme, which is responsible for converting testosterone into the more potent DHT.
- Finasteride: Used to treat Benign Prostatic Hyperplasia (BPH) at a dose of 5mg/day. It effectively reduces prostate volume, improves urinary flow, and lowers DHT levels in the prostate. Side effects can include loss of libido and impotence. It is also used at a lower dose to prevent hair loss.
- Dutasteride: A similar drug that inhibits both type I and type II isoforms of the 5-α-reductase enzyme.
6.3. Androgen Synthesis Inhibitors and Agents with Mixed Mechanisms
- Danazol: This drug has a complex mechanism of action, inhibiting the release of FSH and LH, preventing steroids from binding to their receptors, and inhibiting enzymes involved in steroid synthesis. It has weak androgenic, anabolic, progestational, and glucocorticoid actions. It is used for endometriosis, menorrhagia, fibrocystic breast disease, and hereditary angioneurotic oedema. Side effects include acne, hirsutism, weight gain, and amenorrhea.
6.4. Other Drugs with Anti-Androgenic Activity
Several medications used for other primary indications possess clinically relevant anti-androgenic properties.
- Ketoconazole: An antifungal agent that, at higher doses, inhibits enzymes critical for steroid biosynthesis, thereby reducing the production of testosterone and other androgens.
- Spironolactone: A potassium-sparing diuretic that acts as a competitive antagonist at both aldosterone and androgen receptors, making it useful in treating conditions like hirsutism in women.
- Cimetidine: An H2-receptor antagonist used for acid reflux that has weak anti-androgenic effects and can cause gynecomastia with long-term use.
7. Controlling the Source: GnRH and Gonadotropin Modulators
These drugs act higher up on the HPG axis to control the production of sex hormones.
7.1. Therapeutic Gonadotropins
Preparations of gonadotropins like Menotropins (hMG), Human Chorionic Gonadotropin (hCG), Urofolitropin (FSH), and the recombinant Follitropin beta are used therapeutically to treat infertility, amenorrhoea, and hypogonadism by directly stimulating the gonads.
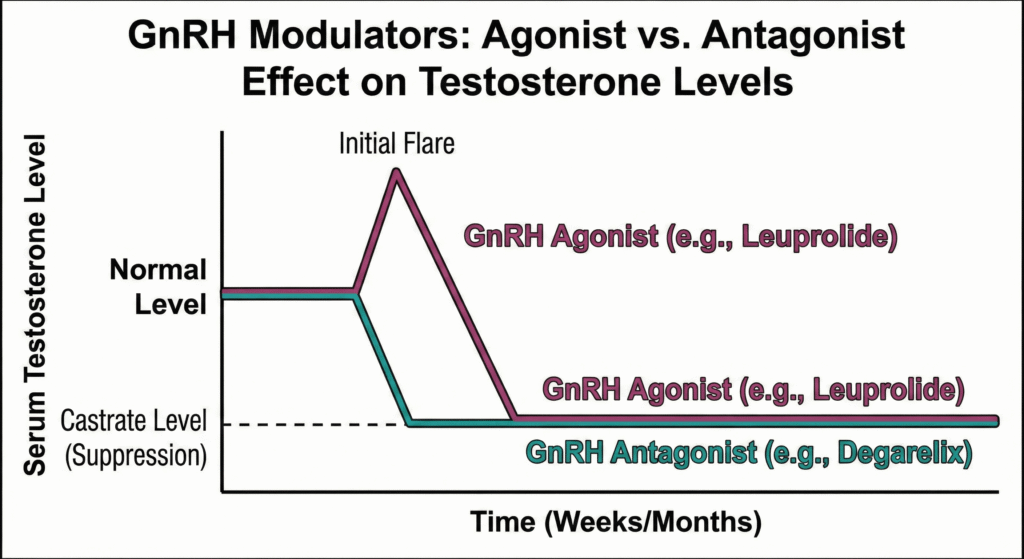
7.2. GnRH Agonists (Superagonists)
This class includes agents like Buserelin, Goserelin, Leuprolide, Nafarelin, Triptorelin, and Histrelin. Their mechanism involves continuous administration, which paradoxically leads to the downregulation and desensitization of GnRH receptors in the pituitary. The result is a profound suppression of LH and FSH secretion, resulting in a state of medical castration (hypogonadism). The resulting profound estrogen and testosterone deficiency is directly responsible for the characteristic adverse effects, including hot flushes, loss of libido, vaginal dryness, and the risk of osteoporosis with long-term use. These agents are used to treat metastatic prostate cancer, endometriosis, precocious puberty, and uterine fibroids.
7.3. GnRH Antagonists
Agents like Ganirelix, Cetrorelix, and Degarelix act as competitive antagonists at the GnRH receptor. This leads to a rapid suppression of gonadotropin release. Their advantages over GnRH agonists include:
- Faster suppression of hormone levels.
- A lower risk of ovarian hyperstimulation syndrome in fertility treatments.
- More complete suppression of gonadotropin secretion. However, pregnancy rates with their use are similar to or may be lower than with agonist protocols.
8. The Future on the Horizon: Selective Androgen Receptor Modulators (SARMs)
Selective Androgen Receptor Modulators (SARMs) are a new, investigational class of drugs designed to selectively target androgen receptors. The goal is to produce the desirable anabolic effects on muscle and bone without the unwanted androgenic side effects on the prostate, skin, and hair.
Potential therapeutic applications being investigated include osteoporosis, muscle wasting, and age-related sarcopenia. Popular examples that have appeared on the market include Ostarine (MK-2866), Ligandrol (LGD-4033), Testolone (RAD-140), and Andarine (S-4).
It is crucial to understand that SARMs are NOT APPROVED BY THE FDA for any clinical use. While they can be legally sold online as “research chemicals,” they cannot be marketed as dietary supplements, and any claims regarding their benefits are not substantiated by regulatory bodies.
9. Conclusion: Key Takeaways on Androgen Pharmacology
The pharmacology of androgens and their modulators is a field of immense therapeutic importance and complexity. From replacing hormonal deficiencies to treating cancer and blocking unwanted masculinizing effects, these drugs intervene at every level of the Hypothalamus-Pituitary-Gonadal axis. The intricate interplay between androgens, their antagonists, and the hormones that regulate them provides a powerful toolkit for clinicians. However, this power comes with a significant risk of adverse effects that demand careful patient selection, monitoring, and management.
Key Takeaways:
- Androgens are more than just masculinizing hormones; they have widespread anabolic, hematopoietic, and metabolic effects, and their primary form, testosterone, serves as a prohormone for both DHT and estradiol.
- Therapeutic delivery is key: Due to high first-pass metabolism, testosterone must be administered via injections, transdermal systems, or as modified oral synthetics, each with distinct pharmacokinetic profiles and risks, such as the hepatotoxicity of 17α-alkylated agents.
- Modulation can occur at multiple levels: Treatment strategies can target androgen receptors directly (antagonists like bicalutamide), inhibit key enzymes (5-α-reductase inhibitors like finasteride), or suppress the entire HPG axis at its source (GnRH modulators like leuprolide).
- Benefit must always be weighed against risk: All androgen-related therapies carry a substantial side effect profile, from virilization in women and gynecomastia in men to adverse cardiovascular and hepatic effects, making contraindications and careful monitoring paramount.
References:
- Brunton LL, Hilal-Dandan R, Knollmann BC. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics. 13th ed. New York: McGraw-Hill Education; 2018.
- Katzung BG. Basic & Clinical Pharmacology. 15th ed. New York: McGraw-Hill Education; 2021.
Short video on this topic:
Androgens-antiandrogens-GnRH-modulators_compressed
Androgens and its modulators
📚 AI Pharma Quiz Generator
🎉 Quiz Results
Medical Disclaimer
The medical information on this post is for general educational purposes only and is provided by Pharmacology Mentor. While we strive to keep content current and accurate, Pharmacology Mentor makes no representations or warranties, express or implied, regarding the completeness, accuracy, reliability, suitability, or availability of the post, the website, or any information, products, services, or related graphics for any purpose. This content is not a substitute for professional medical advice, diagnosis, or treatment; always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition and never disregard or delay seeking professional advice because of something you have read here. Reliance on any information provided is solely at your own risk.