Introduction
The thyroid gland plays a pivotal role in human physiology, modulating metabolism, growth, and development through the production and secretion of thyroid hormones. These hormones, primarily triiodothyronine (T3) and thyroxine (T4), influence virtually every tissue in the body, from skeletal muscle to neural tissue. Understanding thyroid physiology is paramount for grasping how the body maintains metabolic equilibrium, how it responds to external demands, and how disruptions in thyroid function can lead to clinical disorders like hypothyroidism or hyperthyroidism.
In this comprehensive discussion, we will delve into the physiology of the thyroid gland—highlighting its anatomy, embryology, hormone synthesis, storage, release, regulatory mechanisms, actions, and clinical correlations. Drawing from reputed physiology textbooks, such as Guyton & Hall, Ganong’s Review of Medical Physiology, Berne & Levy, and Costanzo Physiology, we aim to provide an in-depth exploration of how the thyroid gland maintains homeostasis and orchestrates myriad physiological processes.
1. Anatomy of the Thyroid Gland
The thyroid gland is a butterfly-shaped endocrine organ located in the anterior region of the neck, just inferior to the larynx. It comprises two lateral lobes connected by a narrow isthmus that typically lies at the level of the second to fourth tracheal rings.
- Size and Weight: In an adult, the thyroid gland typically weighs around 15–20 grams, though size may vary depending on factors like iodine intake and individual physiology.
- Vascular Supply: A prominently vascular gland, it is supplied by the superior and inferior thyroid arteries—branches of the external carotid and subclavian arteries, respectively. This extensive vascularization facilitates efficient hormone release into the bloodstream.
- Microscopic Structure: The functional units of the thyroid gland are thyroid follicles, spherical structures lined by a single layer of follicular cells (thyrocytes) and filled with colloid, a gel-like substance primarily composed of thyroglobulin. Interspersed among the follicles are the parafollicular cells (C cells), which secrete the hormone calcitonin.
1.1 Thyroid Follicles and Colloid
- Follicular (Epithelial) Cells: These cells are responsible for the synthesis, storage, and secretion of thyroid hormones. Their shape (from squamous to columnar) varies according to functional state—more active cells tend to appear columnar.
- Colloid: This acellular material is primarily thyroglobulin, an iodinated glycoprotein serving as a precursor reservoir for T3 and T4. Histologically, colloid staining intensity may reflect the gland’s functional state: a “scalloped” margin suggests active hormone resorption.
1.2 Parafollicular Cells (C Cells)
Although less numerous, parafollicular cells have a crucial role in calcium homeostasis through calcitonin secretion. Calcitonin lowers blood calcium levels by inhibiting osteoclast activity and promoting calcium deposition in bones.
2. Embryological Development
The thyroid gland originates from a midline endodermal proliferation at the base of the tongue, referred to as the foramen cecum. Around the fourth week of gestation, this thyroid primordium descends through the neck via the thyroglossal duct, eventually settling anterior to the trachea. Key embryologic milestones include:
- Thyroglossal Duct Regression: Ideally, the thyroglossal duct involutes by the seventh week; persistent remnants may lead to thyroglossal duct cysts.
- Onset of Hormone Synthesis: Fetal thyroid hormone production begins towards the end of the first trimester, contributing to fetal growth and neurodevelopment.
Embryological anomalies—such as ectopic thyroid tissue—can influence postnatal thyroid function, potentially resulting in hypothyroidism if the ectopic glandular tissue cannot meet the body’s thyroid hormone demands.
3. Synthesis of Thyroid Hormones
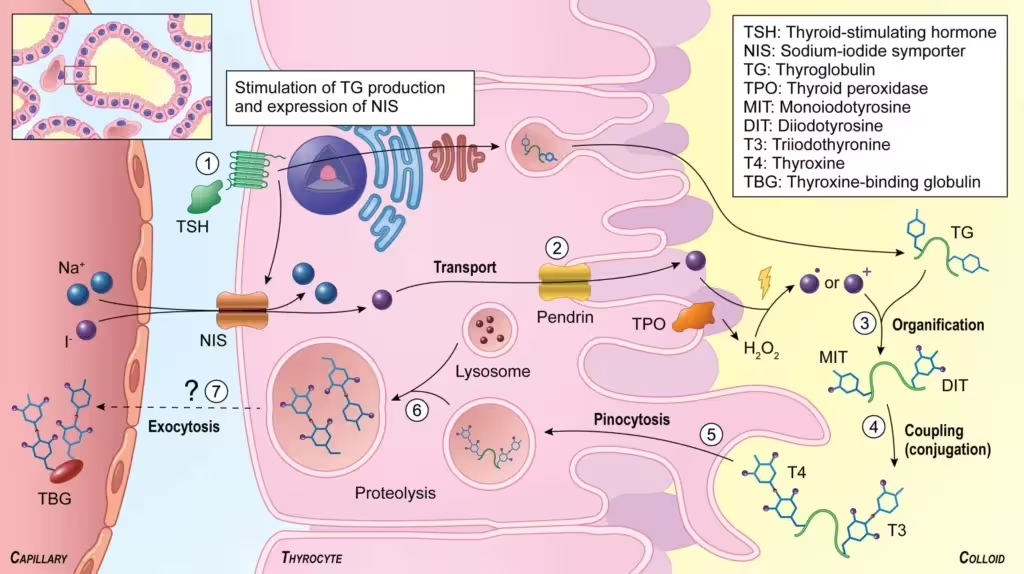
The defining feature of thyroid physiology is the unique mechanism by which the gland incorporates iodine into organic compounds. Synthesizing functional T3 and T4 requires a series of well-regulated steps:
- Iodide Trapping
- Thyroid follicular cells actively transport iodine (as iodide, I⁻) from the bloodstream via a sodium-iodide symporter (NIS) located in the basolateral membrane.
- This process relies on an electrochemical gradient driven by the Na⁺/K⁺-ATPase pump and is regulated by thyroid-stimulating hormone (TSH).
- Oxidation of Iodide
- Upon entering the follicular cell, iodide must be oxidized to an active form by the enzyme thyroid peroxidase (TPO).
- This step converts iodide into an oxidized species (often denoted as I² or I⁺), rendering it reactive enough to bind to tyrosyl residues on thyroglobulin.
- Organification of Iodine
- The newly oxidized iodine is attached to the tyrosine residues of thyroglobulin in the colloid, forming monoiodotyrosine (MIT) and diiodotyrosine (DIT).
- Catalyzed by thyroid peroxidase, this action is described as the organification step.
- Coupling of Iodotyrosines
- DIT and MIT residues couple to form T3 (triiodothyronine) and T4 (tetraiodothyronine or thyroxine). Specifically, T4 arises from coupling two DIT residues, while T3 typically emerges from coupling one MIT with one DIT.
- Storage in Colloid
- Thyroglobulin loaded with T3 and T4 remains stored in the colloid, providing a unique reservoir of thyroid hormone. This storage capacity can meet the body’s hormonal needs for weeks.
- Endocytosis and Secretion
- Stimulated by TSH, follicular cells endocytose thyroglobulin from the colloid. Lysosomal proteases then cleave T3 and T4 from the thyroglobulin backbone.
- Free T3 and T4 are subsequently released into the bloodstream, whereas leftover MIT and DIT are deiodinated to recycle iodide.
3.1 Role of Thyroid Peroxidase
Thyroid peroxidase (TPO) is essential at multiple stages: oxidizing iodide, organizing it onto tyrosine, and coupling iodotyrosines. Autoimmune conditions (e.g., Hashimoto’s thyroiditis) often involve anti-thyroid peroxidase antibodies, undermining TPO function and leading to hypothyroidism.
4. Transport of Thyroid Hormones
Once synthesized, T3 and T4 enter the bloodstream and travel mostly bound to specific carrier proteins:
- Thyroxine-binding globulin (TBG)
- Transthyretin (prealbumin)
- Albumin
These proteins prolong half-life and modulate the bioavailability of thyroid hormones. Only the free fraction (~0.03% of T4 and ~0.3% of T3) is biologically active, exerting negative feedback at the pituitary and hypothalamic levels.
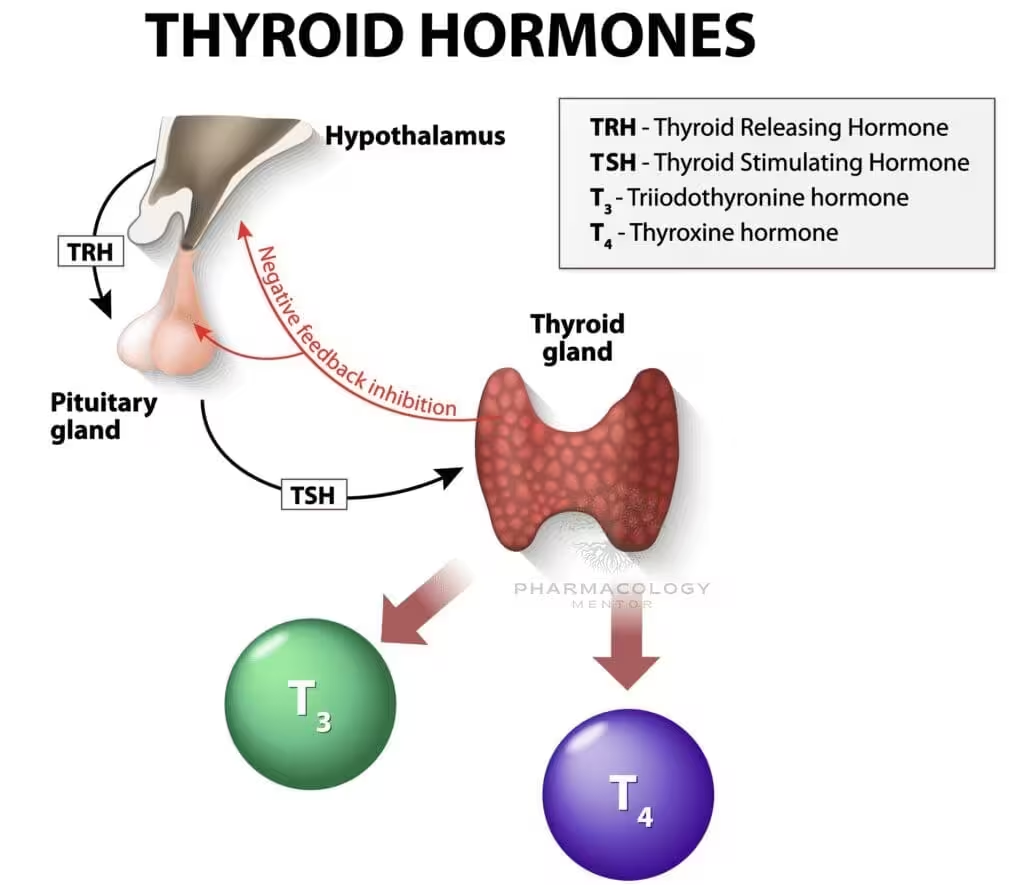
5. Regulation of Thyroid Function
The hypothalamic-pituitary-thyroid (HPT) axis orchestrates thyroid hormone levels:
- Thyrotropin-Releasing Hormone (TRH)
- Synthesized in the hypothalamus and transported to the anterior pituitary, TRH stimulates thyrotroph cells to release TSH.
- Thyroid-Stimulating Hormone (TSH)
- TSH binds to receptors on thyroid follicular cells, increasing cAMP levels, which in turn promotes iodide uptake, thyroglobulin synthesis, and T3/T4 release.
- Negative Feedback
- Elevated circulating T3 and T4 suppress TRH and TSH secretion.
- This negative feedback loop maintains stable plasma levels of thyroid hormones.
5.1 Influence of Other Factors on Thyroid Regulation
- Iodine Levels: Very high iodine intake can trigger the Wolff–Chaikoff effect, transiently reducing thyroid hormone synthesis.
- Pregnancy and Estrogen: Increased TBG levels during pregnancy may increase total T4 while free T4 remains unchanged (if the thyroid gland adapts properly).
- Starvation and Illness: Severe illness and fasting can reduce T3 generation (via decreased peripheral conversion).
6. Metabolism and Peripheral Conversion of T4 to T3
Although circulating T4 is much more abundant than T3 (~90% vs. 10%), T3 is three to four times more potent and is generally considered the active hormone. T4 often serves as a prohormone, undergoing 5’-deiodination in peripheral tissues (liver, kidney, muscle) by iodothyronine deiodinases to form T3.
- Type 1 Deiodinase: Found in the liver, kidney, and thyroid. Produces most of the circulating T3.
- Type 2 Deiodinase: Expressed in the brain, pituitary, and brown adipose tissue, regulating local T3 levels.
- Type 3 Deiodinase: Converts T4 to reverse T3 (rT3) or T3 to T2, effectively inactivating hormones.
7. Mechanism of Action of Thyroid Hormones
Thyroid hormones act through intracellular nuclear receptors, altering gene transcription and protein synthesis:
- Cellular Uptake
- T3 and T4 diffuse across plasma membranes or use specific transporters. Within target cells, T4 is often converted to T3, which binds with higher affinity to the nuclear receptor.
- Nuclear Receptor Binding
- T3 binds to thyroid hormone receptors (TRα and TRβ) located in the cell nucleus.
- In the inactive state, these receptors may form heterodimers with the retinoid X receptor (RXR) bound to thyroid hormone response elements (TREs) on DNA.
- Gene Transcription and Protein Synthesis
- Ligand-bound receptor complexes undergo conformational changes, displacing corepressors and recruiting coactivators that initiate transcription.
- Resulting changes in mRNA and protein expression lead to long-term metabolic and developmental alterations.
7.1 Non-Genomic Effects
Beyond genomic regulation, thyroid hormones can exert non-genomic actions by interacting with cytoplasmic proteins, kinase pathways, or mitochondrial enzymes, eliciting more rapid physiological responses (e.g., modulating ion pumps and kinase signaling).
8. Systemic Actions of Thyroid Hormones
The pleiotropic effects of T3 and T4 influence metabolism, growth, cardiovascular function, neurodevelopment, and more:
8.1 Metabolic Rate and Thermogenesis
- Basal Metabolic Rate (BMR): Thyroid hormones increase BMR by upregulating Na⁺/K⁺-ATPase activity, enhancing oxygen consumption and heat production in tissues such as skeletal muscle, liver, and kidney.
- Nutrient Metabolism: Elevated T3/T4 levels foster carbohydrate and lipid catabolism, accelerating glycogenolysis, gluconeogenesis, lipolysis, and oxidation processes.
- Thermogenesis: In concert with brown adipose tissue, T3 stimulates uncoupling proteins (UCPs), leading to dissipative heat production.
8.2 Growth and Development
- Skeletal Growth: Adequate thyroid hormone is indispensable for normal bone growth and maturation. Children with hypothyroidism may exhibit stunted growth or delayed skeletal development.
- Neural Development: Fetal and neonatal brain maturation—myelination, neuronal differentiation, synapse formation—are critically dependent on thyroid hormones. Congenital hypothyroidism can lead to irreversible intellectual disability if untreated.
8.3 Cardiovascular Effects
- Heart Rate and Contractility: T3 augments β1-adrenergic receptor expression, amplifying responses to catecholamines—increasing heart rate (positive chronotropy), stroke volume, and contractility (positive inotropy).
- Vascular Resistance: Enhanced tissue oxygen consumption often leads to vasodilation and reduced peripheral vascular resistance.
- Blood Pressure: Systolic blood pressure may rise with hyperthyroidism, whereas diastolic pressure can fall.
8.4 Respiratory System
- Thyroid hormones modulate respiratory drive, aiding in maintenance of normal respiratory rates. Enhanced oxygen consumption demands an increase in ventilation to supply tissues and remove carbon dioxide.
8.5 Gastrointestinal System
- Elevated thyroid hormone levels may speed up GI motility, causing diarrhea in hyperthyroid states.
- In contrast, constipation is frequently reported in hypothyroidism.
8.6 Musculoskeletal System
- Protein Turnover: T3 accelerates both protein synthesis and breakdown; net balance depends on the hormone level. Excess leads to muscle wasting over time.
- Bone: Excess thyroid hormone can increase bone resorption, raising the risk for osteoporosis if hyperthyroidism persists.
8.7 Reproductive System
- Thyroid status profoundly impacts fertility and menstrual cycles: hypothyroidism might contribute to menorrhagia or amenorrhea, while hyperthyroidism can cause oligomenorrhea.
8.8 Central Nervous System (CNS)
- Mood and Cognition: Hyperthyroidism is associated with nervousness, anxiety, and hyperreflexia, whereas hypothyroidism correlates with lethargy, depression, and slowed reflexes.
- Sympathetic Nervous System: Thyroid hormones synergize with catecholamines, explaining many hypermetabolic and adrenergic symptoms in hyperthyroid patients.
9. Pathophysiological Considerations
9.1 Hypothyroidism
Characterized by inadequate thyroid hormone secretion:
- Primary Hypothyroidism
- Often due to Hashimoto’s thyroiditis, iodine deficiency, or post-thyroidectomy.
- Laboratory findings feature elevated TSH and low T4.
- Clinical manifestations: fatigue, weight gain, bradycardia, cold intolerance, menorrhagia, dry skin, coarse hair, and potential dyslipidemia.
- Congenital Hypothyroidism
- Untreated can lead to cretinism, encompassing mental retardation and stunted growth.
- Neonatal screening is critical for early detection and thyroid hormone replacement.
9.2 Hyperthyroidism
Excessive thyroid hormone production:
- Graves’ Disease
- An autoimmune disorder characterized by thyroid-stimulating immunoglobulins (TSI) that activate the TSH receptor.
- Common clinical signs include goiter, exophthalmos, palpitations, heat intolerance, and weight loss.
- Laboratory values indicate decreased TSH and elevated T3/T4.
- Thyrotoxicosis
- A hypermetabolic state arising from excessive circulating thyroid hormones, which can occur via Graves’ disease or from thyroiditis where stored hormone is released.
9.3 Iodine-Related Disorders
- Iodine Deficiency Goiter
- Lack of iodine impairs hormone synthesis, prompting TSH elevations, leading to follicular cell hyperplasia and an enlarged thyroid (goiter).
- Wolff–Chaikoff Effect
- Sudden exposure to excess iodine triggers transient inhibition of TPO, reducing thyroid hormone output.
10. Assessment of Thyroid Function
Healthcare practitioners commonly evaluate the thyroid axis through laboratory tests:
- TSH (Thyroid-Stimulating Hormone)
- A sensitive marker for primary thyroid dysfunction: elevated TSH in hypothyroidism, suppressed TSH in hyperthyroidism.
- Free T4 and Free T3
- Provide direct measures of active hormone levels.
- T4 generally more abundant, while T3 is more metabolically potent.
- Thyroid Autoantibodies
- Anti-thyroid peroxidase (anti-TPO): prevalent in Hashimoto’s thyroiditis.
- Thyroid-stimulating immunoglobulin (TSI): hallmark of Graves’ disease.
- Imaging
- Ultrasound to assess gland morphology.
- Radioactive Iodine Uptake (RAIU) distinguishing hyperthyroid etiologies (e.g., Graves’ vs. thyroiditis).
- Clinical Signs and Symptoms
- Vital clues: heart rate, weight changes, reflex speeds, hair/skin changes, and subjective complaints guide further testing.
11. Thyroid Hormone Replacement and Pharmacological Modulation
Several medications impact thyroid hormone synthesis or catabolism:
- Levothyroxine (T4)
- Mainstay therapy for hypothyroidism, mimicking endogenous T4.
- The body peripherally converts T4 to T3 as needed.
- Dosage adjustments are TSH-guided.
- Liothyronine (T3)
- Occasionally used when rapid correction required, though fluctuating levels can cause side effects.
- Anti-Thyroid Drugs
- Methimazole and Propylthiouracil (PTU) block TPO. PTU also inhibits peripheral T4-to-T3 conversion.
- Used in hyperthyroidism management, including Graves’ disease.
- Beta-Blockers
- E.g., Propranolol used to manage adrenergic symptoms in hyperthyroidism by reducing T4-to-T3 conversion.
- Iodine
- In large doses (Lugol’s iodine), temporarily suppresses hormone release (Wolff–Chaikoff effect) before eventual escape phenomenon.
12. Environmental and Nutritional Influences
- Iodine Intake: Adequate iodine is crucial—both deficiency and excess can provoke dysfunction. Public health measures like iodized salt aim to reduce goiter prevalence.
- Goitrogens: Certain foods (cruciferous vegetables like cabbage, broccoli) contain goitrin or related substances that may interfere with thyroid hormone synthesis if consumed excessively.
13. Newer Insights: Thyroid Hormones in Nonthyroidal Illness
In severe systemic illness (e.g., critical care patients), an “euthyroid sick syndrome” or “nonthyroidal illness syndrome (NTIS)” frequently emerges, characterized by low T3, normal or low T4, and normal or low TSH. This condition likely reflects an adaptive response, with decreased peripheral conversion of T4 to T3 and altered regulation. While it was once believed to require T3 supplementation, current consensus holds that treatment of the underlying illness is more crucial.
14. Future Directions in Thyroid Physiology Research
Continuing research seeks to refine understanding of thyroid hormone metabolism and action:
- Thyroid Hormone Analogs: Investigated for cholesterol-lowering or weight-loss properties, selectively harnessing metabolic benefits without adverse cardiac effects.
- Molecular Genetics: Identifying polymorphisms in deiodinases or thyroid hormone receptors could offer personalized treatment strategies.
- Non-Genomic Effects: Elucidating the precise molecular cascades of rapid T3 signaling may lead to novel therapeutic avenues for metabolic disorders.
15. Clinical Correlations and Practical Applications
15.1 Critical Developmental Windows
During embryonic and early neonatal life, adequate thyroid hormones are vital for brain, bone, and somatic development. Screening programs for congenital hypothyroidism are crucial for preventing irreversible neurocognitive deficits.
15.2 Thyroid Emergencies
- Myxedema Coma: Extreme hypothyroidism presenting with hypothermia, coma, and cardiovascular collapse.
- Thyroid Storm: A hyperthyroid crisis characterized by fever, tachyarrhythmias, and potential organ failure, demanding immediate medical intervention.
15.3 Thyroid Nodules and Cancer
Although mostly benign, thyroid nodules can occasionally harbor malignancies, with papillary carcinoma being the most common. Understanding nodular physiology, TSH regulation, and uptake scans are pivotal in diagnosis and management.
16. Summary and Key Takeaways (SEO-Optimized Conclusion)
The thyroid gland is a cornerstone of endocrine regulation, influencing metabolic rate, growth, development, and various organ systems throughout the body. By synthesizing T3 and T4—via iodide trapping, thyroglobulin iodination, and hormone coupling—and meticulously regulating their release under the hypothalamic-pituitary-thyroid axis, the thyroid gland ensures homeostatic balance. Meticulous involvement in energy balance, thermoregulation, cardiovascular function, neurological development, and skeletal growth underscores the breadth of thyroid hormone action.
Thyroid physiology is further nuanced by negative feedback loops, peripheral conversion of T4 to T3, and complex interactions with binding proteins like thyroxine-binding globulin (TBG). Hyperthyroidism and hypothyroidism serve as prime examples of the clinical importance of thyroid function, shaping everything from basal metabolic rate to emotional well-being. Meanwhile, environmental elements, including iodine intake, can profoundly affect thyroid hormone synthesis and secretion.Continued research promises deeper insights into the non-genomic roles of thyroid hormones, the intricacies of deiodinase polymorphisms, and the development of novel therapeutic agents that harness or modify thyroid hormone pathways. As physicians, physiologists, and researchers strive to refine diagnostic tools and treatments, our expanding grasp of thyroid physiology remains indispensable for safeguarding individual health—from neonatal development to advanced age.
References
Costanzo LS. Costanzo Physiology. 6th ed. Philadelphia, PA: Elsevier; 2018.
Hall JE, Guyton AC. Guyton and Hall Textbook of Medical Physiology. 13th ed. Philadelphia, PA: Elsevier; 2016.
Barrett KE, Barman SM, Boitano S, Brooks HL. Ganong’s Review of Medical Physiology. 25th ed. New York, NY: McGraw-Hill; 2016.
Koeppen BM, Stanton BA. Berne & Levy Physiology. 7th ed. Philadelphia, PA: Elsevier; 2017.
Medical Disclaimer
The medical information on this post is for general educational purposes only and is provided by Pharmacology Mentor. While we strive to keep content current and accurate, Pharmacology Mentor makes no representations or warranties, express or implied, regarding the completeness, accuracy, reliability, suitability, or availability of the post, the website, or any information, products, services, or related graphics for any purpose. This content is not a substitute for professional medical advice, diagnosis, or treatment; always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition and never disregard or delay seeking professional advice because of something you have read here. Reliance on any information provided is solely at your own risk.

