1. Introduction to Lipoproteins and Lipid Metabolism
Hyperlipidemia (or dyslipidemia) is defined as an elevation in plasma lipids, including cholesterol, cholesterol esters, triglycerides (TGs), and phospholipids. These lipids are insoluble in water and must be transported in the blood complexed with specialized proteins known as apoproteins (apolipoproteins). The lipid-protein complex is called a lipoprotein.
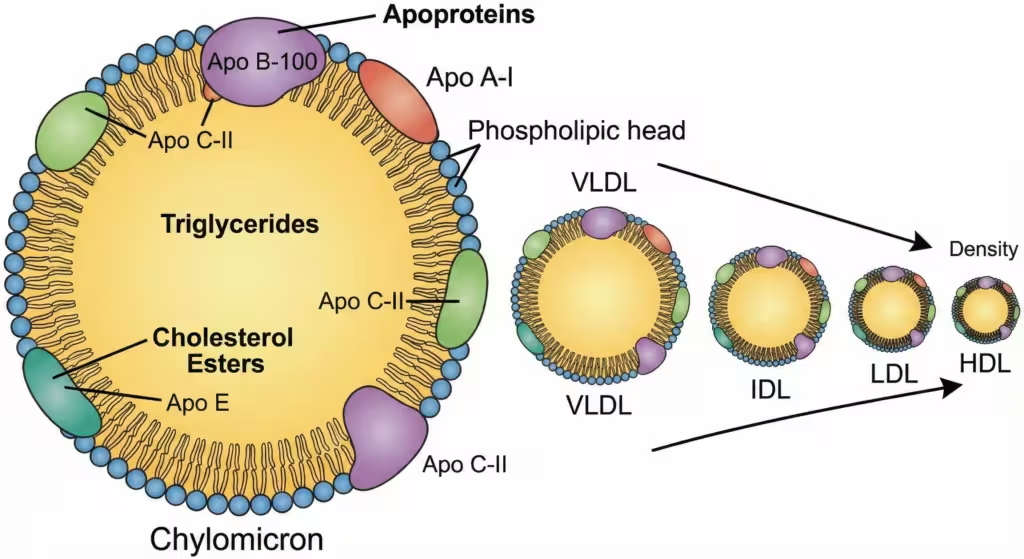
Figure 1. Structure and classification of lipoproteins. Lipoproteins consist of a hydrophobic core of triglycerides and cholesteryl esters surrounded by a hydrophilic shell of phospholipids, free cholesterol, and apoproteins. They are classified by density and size, from the large, TG-rich chylomicrons to the small, dense, protein-rich HDL.
Dyslipidemia is a major risk factor for atherosclerosis and atherosclerosis-associated conditions such as coronary artery disease (CAD), ischemic stroke, and peripheral vascular disease.
1.1 Classification of Lipoproteins
Lipoproteins are classified based on their density, size, and composition. The “Good” vs. “Bad” cholesterol dichotomy is central to clinical pharmacology.
| Lipoprotein | Density | Major Lipid Component | Source | Major Apoproteins | Function |
| Chylomicrons | Lowest | Triglycerides (Dietary) | Intestine | B-48, C-II, E | Transport dietary TGs to adipose/muscle tissue. |
| VLDL (Very Low Density) | Low | Triglycerides (Endogenous) | Liver | B-100, C-II, E | Transport hepatic TGs to peripheral tissues. |
| IDL (Intermediate Density) | Intermediate | Cholesterol & TGs | Catabolism of VLDL | B-100, E | Precursor to LDL. |
| LDL (Low Density) | Low | Cholesterol | Catabolism of IDL | B-100 | “Bad Cholesterol”: Transports cholesterol to tissues. Highly atherogenic. |
| HDL (High Density) | Highest | Phospholipids & Cholesterol | Liver/Intestine | A-I, A-II, C-II, E | “Good Cholesterol”: Reverse cholesterol transport (tissues to liver). |
Note for Exams:
- Apo B-100 is the ligand for the LDL receptor.
- Apo C-II is the cofactor for Lipoprotein Lipase (LPL).
- Apo A-I activates LCAT (Lecithin-Cholesterol Acyltransferase) for cholesterol esterification in HDL.
2. Classification of Hypolipidemic Drugs
Drugs used to treat dyslipidemia can be classified based on their primary mechanism of action, which involves different organs and pathways.
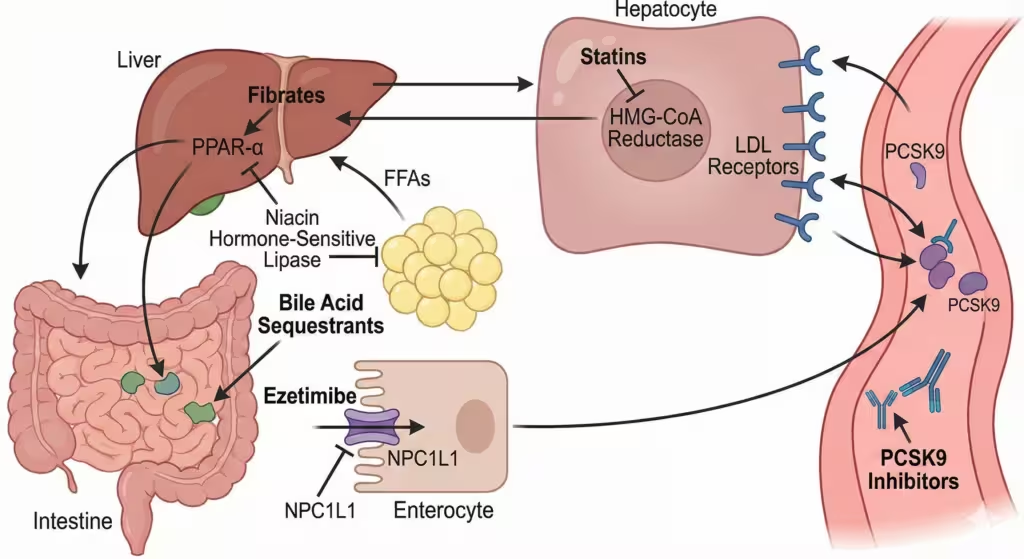
Figure 2. Sites of action of major antihyperlipidemic drugs. The diagram illustrates the key organs (liver, intestine, adipose tissue) and the specific molecular targets for statins, fibrates, bile acid sequestrants, ezetimibe, niacin, and PCSK9 inhibitors.
- HMG-CoA Reductase Inhibitors (Statins):
- Examples: Atorvastatin, Rosuvastatin, Simvastatin, Lovastatin, Pravastatin, Fluvastatin, Pitavastatin.
- Bile Acid Sequestrants (Resins):
- Examples: Cholestyramine, Colestipol, Colesevelam.
- Cholesterol Absorption Inhibitors:
- Examples: Ezetimibe.
- Fibric Acid Derivatives (Fibrates):
- Examples: Fenofibrate, Gemfibrozil, Bezafibrate.
- PCSK9 Inhibitors (Monoclonal Antibodies):
- Examples: Evolocumab, Alirocumab.
- Nicotinic Acid (Niacin):
- Examples: Crystalline Niacin, Extended-release Niacin.
- ATP-Citrate Lyase (ACL) Inhibitors:
- Examples: Bempedoic Acid.
- Microsomal Triglyceride Transfer Protein (MTP) Inhibitors:
- Examples: Lomitapide (restricted use for Homozygous Familial Hypercholesterolemia – HoFH).
- Antisense Oligonucleotides:
- Examples: Mipomersen (targets Apo B-100 synthesis).
3. HMG-CoA Reductase Inhibitors (Statins)
“The cornerstone of modern lipid-lowering therapy.”
3.1 Mechanism of Action
Statins are structural analogs of HMG-CoA (3-hydroxy-3-methylglutaryl-coenzyme A). Their primary action is in the liver.
- Competitive Inhibition: They competitively inhibit the enzyme HMG-CoA Reductase, which catalyzes the rate-limiting step in cholesterol synthesis (conversion of HMG-CoA to Mevalonate).
- Up-regulation of LDL Receptors: The depletion of intracellular hepatic cholesterol pools triggers a signaling pathway involving the SREBP (Sterol Regulatory Element-Binding Protein) transcription factor.
- Result: SREBP translocates to the nucleus and up-regulates the transcription of the LDL receptor gene. Increased expression of LDL receptors on the hepatocyte surface leads to increased clearance of LDL and VLDL remnants from the blood.
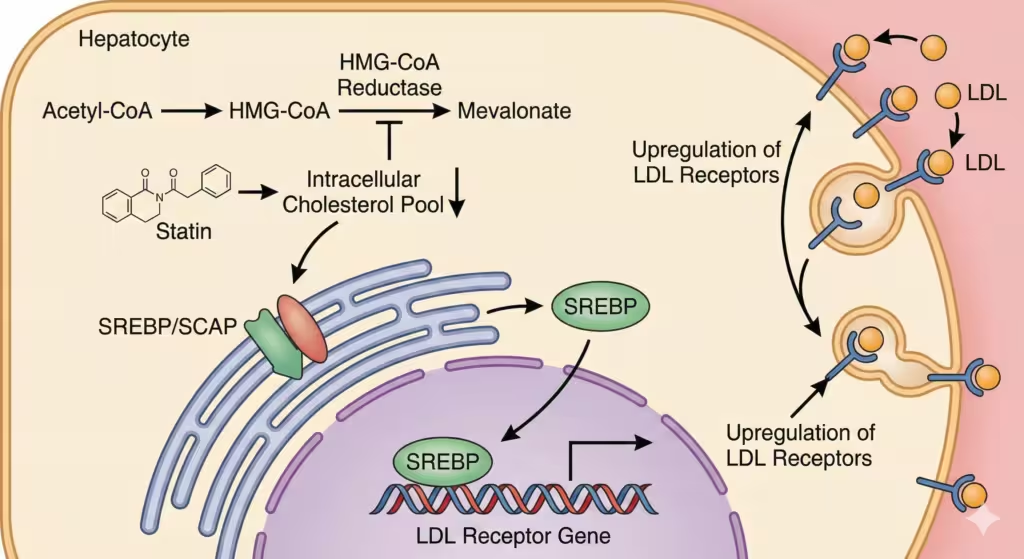
Figure 3. Mechanism of action of statins. Statins inhibit HMG-CoA reductase, the rate-limiting enzyme in cholesterol synthesis. This lowers intracellular cholesterol, activating the SREBP pathway. SREBP enters the nucleus and increases the expression of the LDL receptor gene, leading to more LDL receptors on the cell surface and increased clearance of LDL from the blood.
Effect on Lipid Profile:
- LDL: ↓ 20–60% (Most potent agents).
- TG: ↓ 10–30%.
- HDL: ↑ 5–15%.
3.2 Pleiotropic Effects (Non-Lipid Effects)
Statins provide cardiovascular benefits beyond lipid lowering (often asked in PG exams):
- Improvement of endothelial function (increased NO synthesis).
- Stabilization of atherosclerotic plaques.
- Anti-inflammatory effects (reduction in CRP).
- Inhibition of platelet aggregation.
3.3 Pharmacokinetics (The “Statins Table”)
| Drug | Lipophilicity | Prodrug? | Metabolism (CYP) | Half-life (t1/2) | Timing of Dose |
| Lovastatin | High | Yes (Lactone) | 3A4 | 2–4 hrs | Evening/Meal |
| Simvastatin | High | Yes (Lactone) | 3A4 | 2–3 hrs | Evening |
| Pravastatin | Low (Hydrophilic) | No | Sulfation (Not CYP) | 1–3 hrs | Bedtime |
| Atorvastatin | High | No | 3A4 | 14 hrs | Anytime |
| Rosuvastatin | Low (Hydrophilic) | No | 2C9 (Minor) | 19 hrs | Anytime |
| Fluvastatin | High | No | 2C9 | 1–3 hrs | Bedtime |
| Pitavastatin | High | No | 2C9 | 12 hrs | Anytime |
- Note: Atorvastatin and Rosuvastatin have long half-lives and can be taken at any time of day. Short-acting statins (Simvastatin, Lovastatin) are best taken at night when hepatic cholesterol synthesis peaks.
3.4 Adverse Effects
- Hepatotoxicity: Elevated serum transaminases (ALT/AST). Usually reversible. Monitor at baseline.
- Myopathy and Rhabdomyolysis:
- Manifests as muscle pain, weakness, and elevated Creatine Kinase (CK).
- Risk Factors: Old age, renal insufficiency, hypothyroidism, and interaction with CYP3A4 inhibitors.
- Cerivastatin was withdrawn due to severe rhabdomyolysis.
- New-Onset Diabetes: Slight increase in HbA1c (benefit still outweighs risk in CVD patients).
3.5 Drug Interactions
- CYP3A4 Inhibitors: Macrolides (Erythromycin), Azole antifungals (Ketoconazole), HIV protease inhibitors, and Grapefruit juice increase levels of Lovastatin, Simvastatin, and Atorvastatin, increasing myopathy risk.
- Gemfibrozil + Statins: Increases risk of myopathy (inhibits glucuronidation of statins). Fenofibrate is safer if combination is needed.
4. Fibric Acid Derivatives (Fibrates)
Primary Indication: Severe Hypertriglyceridemia.
4.1 Mechanism of Action
Fibrates are agonists of PPAR-α (Peroxisome Proliferator-Activated Receptor-alpha), a nuclear transcription factor.
- Increased LPL Activity: PPAR-α activation increases the expression of Lipoprotein Lipase (LPL), enhancing the clearance of TG-rich lipoproteins (VLDL, Chylomicrons).
- Increased Fatty Acid Oxidation: Promotes beta-oxidation of fatty acids in the liver, reducing VLDL synthesis.
- Increased HDL: Increases synthesis of Apo A-I and Apo A-II.
Effect on Lipid Profile:
- TG: ↓ 40–50% (Most effective drugs for TGs).
- LDL: Variable (Can sometimes increase LDL in patients with very high TGs).
- HDL: ↑ 10–20%.
4.2 Adverse Effects
- Gastrointestinal: Dyspepsia, abdominal pain (most common).
- Gallstones (Cholelithiasis): Fibrates increase cholesterol excretion in bile, leading to lithogenicity.
- Myopathy: Risk increases if combined with statins.
4.3 Contraindications
- Severe renal impairment.
- Pre-existing gallbladder disease.
- Pregnancy.
5. Bile Acid Sequestrants (Resins)
Drugs: Cholestyramine, Colestipol, Colesevelam.
5.1 Mechanism of Action
- These are large, positively charged, non-absorbable polymers.
- They bind negatively charged bile acids in the intestine, forming an insoluble complex excreted in feces.
- Interruption of Enterohepatic Circulation: This prevents the reabsorption of bile acids.
- Compensatory Response: The liver must convert more cholesterol into bile acids to replenish the pool. This lowers hepatic cholesterol content → Upregulation of LDL receptors → Lower plasma LDL.
5.2 Clinical Nuance
- Can transiently increase Triglycerides (via increased VLDL synthesis). Therefore, contraindicated in patients with hypertriglyceridemia (>400 mg/dL).
5.3 Adverse Effects
- GI Issues: Bloating, constipation, flatulence (poor compliance). Colesevelam is better tolerated.
- Drug Interactions: They bind and decrease absorption of many drugs (Digoxin, Warfarin, Thyroxine, Thiazides) and fat-soluble vitamins (A, D, E, K).
- Counseling: Take other medications 1 hour before or 4 hours after taking resins.
6. Cholesterol Absorption Inhibitors
Drug: Ezetimibe.
6.1 Mechanism of Action
- Selectively inhibits the intestinal absorption of dietary and biliary cholesterol at the brush border of the small intestine.
- Target: It blocks the NPC1L1 (Niemann-Pick C1-Like 1) transport protein.
- Reduces delivery of intestinal cholesterol to the liver.
6.2 Clinical Use
- Usually used as an add-on to statins (e.g., Atorvastatin + Ezetimibe).
- The combination allows for greater LDL reduction without increasing the statin dose (“Save the liver/muscle” strategy).
- IMPROVE-IT Trial: Showed that adding Ezetimibe to Simvastatin further reduced cardiovascular events compared to statin alone.
7. Nicotinic Acid (Niacin)
“The most effective agent for raising HDL.”
7.1 Mechanism of Action
- Inhibits hormone-sensitive lipase in adipose tissue → Reduces breakdown of TGs to Free Fatty Acids (FFA).
- Reduced flux of FFAs to the liver → Decreased hepatic synthesis of TGs and VLDL.
- Since LDL is derived from VLDL, LDL levels also fall.
- Increases the half-life of Apo A-I, significantly raising HDL.
7.2 Adverse Effects & Limitations
- Cutaneous Flushing: Intense warmth/itching primarily on the face and upper trunk. Mediated by Prostaglandin D2. Can be blunted by taking Aspirin 30 mins prior.
- Hyperglycemia: Worsens insulin resistance (Caution in Diabetics).
- Hyperuricemia: Can precipitate gout.
- Hepatotoxicity: More common with sustained-release preparations.
Note: Due to side effects and lack of robust outcomes data in the statin era (AIM-HIGH, HPS2-THRIVE trials), Niacin use has declined significantly.
8. PCSK9 Inhibitors (The New Era)
Drugs: Alirocumab, Evolocumab (Injectable monoclonal antibodies).
8.1 Mechanism of Action
- Physiology: PCSK9 (Proprotein Convertase Subtilisin/Kexin type 9) is an enzyme that binds to LDL receptors and promotes their lysosomal degradation, preventing them from recycling back to the cell surface.
- Drug Action: These antibodies bind to PCSK9, preventing it from binding to the LDL receptor.
- Result: More LDL receptors are available on the liver surface to clear LDL from the blood.
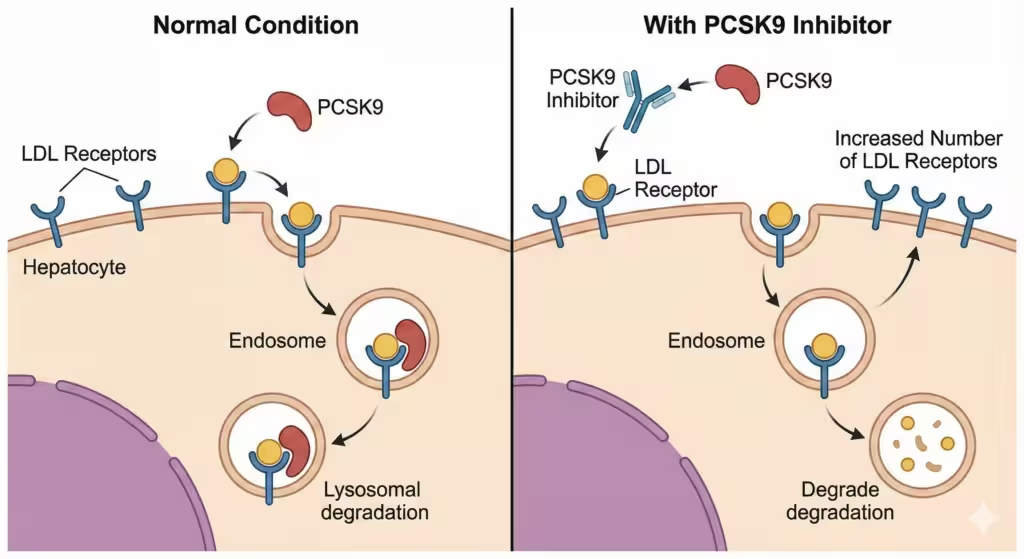
Figure 4. Mechanism of action of PCSK9 inhibitors. In the normal state, PCSK9 binds to the LDL receptor and promotes its lysosomal degradation. PCSK9 inhibitors (monoclonal antibodies) bind to circulating PCSK9, preventing it from binding to the receptor. This allows the LDL receptor to recycle back to the cell surface, increasing the number of receptors available to clear LDL.
8.2 Efficacy
- Potent LDL reduction (↓ 50–70% on top of statins).
- Proven to reduce cardiovascular events (FOURIER, ODYSSEY OUTCOMES trials).
8.3 Clinical Use
- Familial Hypercholesterolemia (HeFH or HoFH).
- Patients with ASCVD who cannot achieve LDL goals on max-tolerated statins.
- Statin-intolerant patients.
9. Newer and Miscellaneous Agents
9.1 Bempedoic Acid
- Mechanism: Inhibits ATP-citrate lyase (ACL), an enzyme upstream of HMG-CoA reductase in the cholesterol synthesis pathway.
- Unique Feature: It is a prodrug activated only in the liver (by ACSVL1), not in skeletal muscle. Therefore, it has a lower risk of muscle-related side effects compared to statins.
- Use: Statin-intolerant patients.
9.2 Lomitapide
- Mechanism: Inhibitor of Microsomal Triglyceride Transfer Protein (MTP). MTP is required for the assembly of VLDL and Chylomicrons.
- Use: Homozygous Familial Hypercholesterolemia (HoFH).
- Toxicity: High risk of hepatotoxicity (fatty liver). RESTRICTED program.
9.3 Omega-3 Fatty Acids (EPA/DHA)
- Products: Icosapent ethyl (highly purified EPA).
- Mechanism: Reduces hepatic VLDL synthesis.
- Use: Severe hypertriglyceridemia. The REDUCE-IT trial showed cardiovascular benefit with high-dose Icosapent ethyl.
10. Comparative Summary Table for Exam Revision
| Drug Class | Primary Effect | LDL | HDL | TG | Major Side Effects |
| Statins | ↓ LDL synthesis | ↓↓↓ | ↑ | ↓ | Myopathy, ↑ Liver enzymes, Diabetes risk. |
| Fibrates | ↑ TG clearance | ↓ or ↔ | ↑↑ | ↓↓↓ | Dyspepsia, Gallstones, Myopathy. |
| Niacin | ↓ VLDL secretion | ↓↓ | ↑↑↑ | ↓↓ | Flushing, Gout, Hyperglycemia. |
| Resins | ↑ Bile excretion | ↓↓ | ↔ | ↑ (bad) | GI distress, bloating, constipation. |
| Ezetimibe | ↓ Chol. absorption | ↓↓ | ↔ | ↓ | Diarrhea, rare liver enzyme elevation. |
| PCSK9-i | ↑ LDL recycling | ↓↓↓↓ | ↑ | ↓ | Injection site reactions, Cost. |
11. Clinical Management Guidelines (A Brief Overview)
Current guidelines (e.g., ACC/AHA 2018, ESC 2019) move away from “target numbers” alone and focus on Risk Stratification.
- ASCVD (Atherosclerotic Cardiovascular Disease) Group: History of MI, Stroke, PAD.
- High Intensity Statin (Atorvastatin 40-80mg, Rosuvastatin 20-40mg) is mandatory.
- Severe Hypercholesterolemia: LDL ≥ 190 mg/dL.
- High Intensity Statin.
- Diabetes Mellitus: Age 40–75 with LDL ≥ 70 mg/dL.
- Moderate to High Intensity Statin.
- Primary Prevention: Calculate 10-year ASCVD risk. If risk > 7.5%, start statin.
12. References
- Brunton LL, Hilal-Dandan R, Knollmann BC. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics. 13th ed. New York: McGraw-Hill Education; 2018.
- Katzung BG. Basic & Clinical Pharmacology. 15th ed. New York: McGraw-Hill Education; 2021.
- Ritter JM, Flower R, Henderson G, Loke YK, MacEwan D, Rang HP. Rang & Dale’s Pharmacology. 9th ed. Edinburgh: Elsevier; 2020.
- Whalen K. Lippincott Illustrated Reviews: Pharmacology. 7th ed. Philadelphia: Wolters Kluwer; 2019.
- Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. J Am Coll Cardiol. 2019;73(24):e285-e350.
📚 AI Pharma Quiz Generator
🎉 Quiz Results
Medical Disclaimer
The medical information on this post is for general educational purposes only and is provided by Pharmacology Mentor. While we strive to keep content current and accurate, Pharmacology Mentor makes no representations or warranties, express or implied, regarding the completeness, accuracy, reliability, suitability, or availability of the post, the website, or any information, products, services, or related graphics for any purpose. This content is not a substitute for professional medical advice, diagnosis, or treatment; always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition and never disregard or delay seeking professional advice because of something you have read here. Reliance on any information provided is solely at your own risk.