1. Introduction: The Incretin Effect
To understand GLP-1 agonists, one must first understand the “Incretin Effect.” This physiological phenomenon describes the observation that oral glucose administration elicits a much higher insulin response than an isoglycemic intravenous (IV) glucose infusion.
- Definition: The gut-derived amplification of insulin secretion.
- Mediators: This effect is mediated by two hormones secreted by the gut mucosa, known as Incretins:
- GLP-1 (Glucagon-Like Peptide-1): Secreted by L-cells in the ileum and colon.
- GIP (Glucose-dependent Insulinotropic Polypeptide): Secreted by K-cells in the duodenum and jejunum.
In Type 2 Diabetes Mellitus (T2DM), the incretin effect is significantly blunted, predominantly due to a reduction in GLP-1 secretion or responsiveness. This provides the rationale for pharmacological replacement.
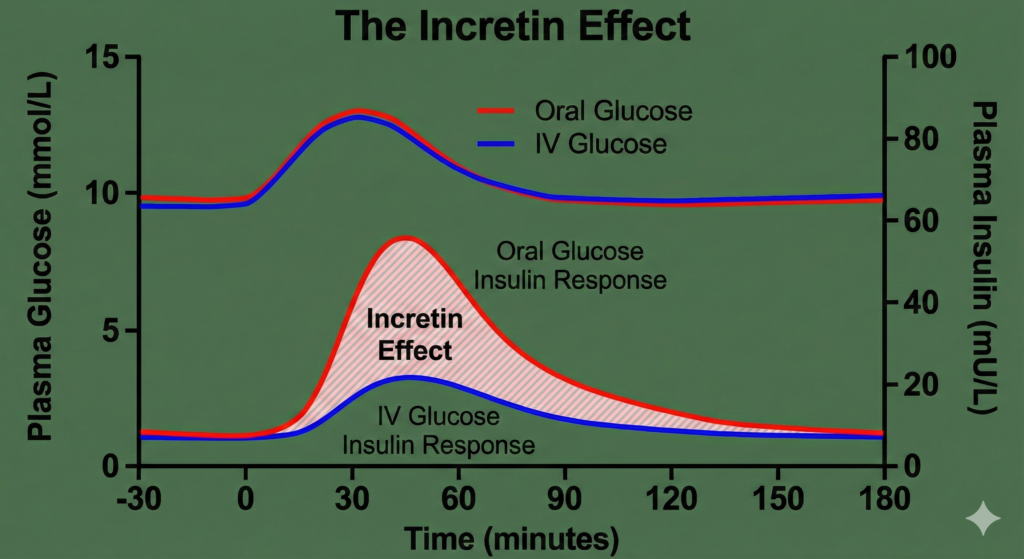
Figure 1. The Incretin Effect. The graph demonstrates the difference in plasma insulin levels following oral glucose intake versus intravenous infusion, despite identical plasma glucose levels. This difference is attributed to the release of incretin hormones (GLP-1 and GIP).
2. Physiology of Endogenous GLP-1
Endogenous GLP-1 is rapidly degraded (half-life < 2 minutes) by the enzyme Dipeptidyl Peptidase-4 (DPP-4). This rapid degradation makes native GLP-1 unsuitable as a drug.
Physiological Actions of GLP-1:
- Pancreas (Beta Cells): Stimulates insulin secretion in a glucose-dependent manner (only works when blood sugar is high).
- Pancreas (Alpha Cells): Suppresses inappropriate glucagon secretion.
- Stomach: Slows gastric emptying, which blunts the post-prandial glucose spike.
- Brain (Hypothalamus): Promotes satiety and reduces appetite.
3. Classification of GLP-1 Receptor Agonists
Pharmacological agents are structurally modified to resist degradation by DPP-4, prolonging their half-life. They are classified based on their structure and duration of action.
3.1 Based on Structure
- Exendin-4 Based (Gila Monster Saliva Analogs):
- Exenatide (Synthetic Exendin-4).
- Lixisenatide.
- Note: Lower homology (~50%) to human GLP-1; higher risk of antibody formation.
- Human GLP-1 Analogs:
- Liraglutide (97% homology).
- Semaglutide (94% homology).
- Dulaglutide.
- Note: Modified with fatty acid chains to bind albumin, protecting them from DPP-4 and reducing renal clearance.
3.2 Based on Duration of Action
| Category | Drugs | Dosing Frequency | Effect on Fasting vs. Post-Prandial Glucose (PPG) |
| Short-Acting | Exenatide (Standard), Lixisenatide | Twice Daily / Daily | Predominantly lowers PPG (via delayed gastric emptying). |
| Long-Acting | Liraglutide, Lixisenatide | Daily | Lowers both Fasting and PPG. |
| Ultra-Long Acting | Semaglutide, Dulaglutide, Exenatide XR | Once Weekly | Strong reduction in Fasting Glucose; sustained control. |
4. Mechanism of Action
GLP-1 agonists bind to the GLP-1 receptor, a G-protein coupled receptor (GPCR) on the surface of pancreatic beta-cells and other tissues.
Intracellular Signaling:
- Binding activates Adenylyl Cyclase.
- Increases intracellular cAMP.
- Activates Protein Kinase A (PKA) and Epac2.
- Closes KATP channels and opens voltage-gated Ca2+ channels.
- Result: Exocytosis of insulin granules.
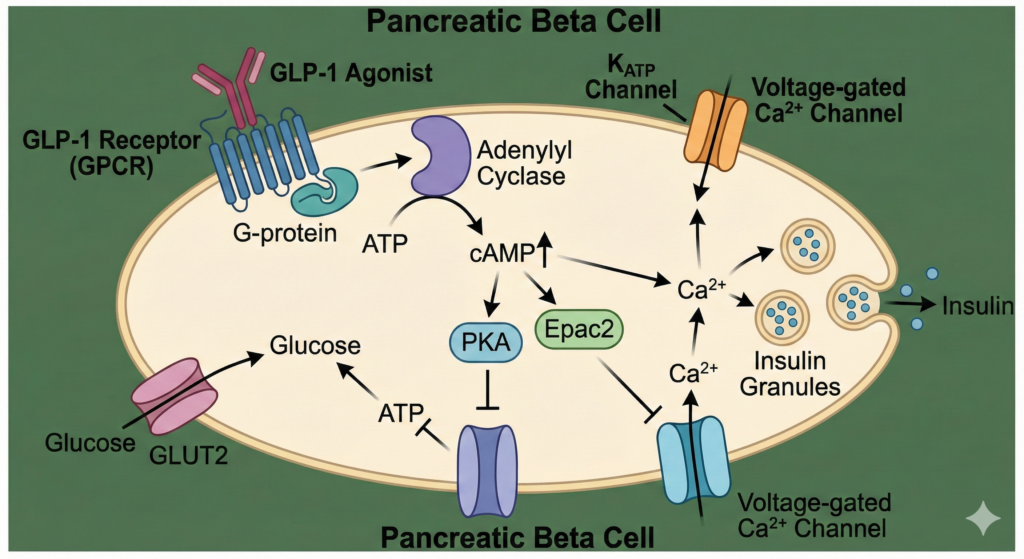
Figure 2. Molecular Mechanism. GLP-1 binds to its GPCR on the beta-cell, increasing cAMP. This pathway enhances glucose-dependent insulin secretion. Crucially, this pathway is only active when glucose enters the cell, minimizing the risk of hypoglycemia.
Systemic Effects ( The “Pleiotropic” Effects):
- Weight Loss: Via central signaling in the hypothalamus (POMC/CART neurons) increasing satiety.
- Cardiovascular: Improvement in endothelial function, reduction in systolic BP, and reduction in atherosclerotic plaque progression.
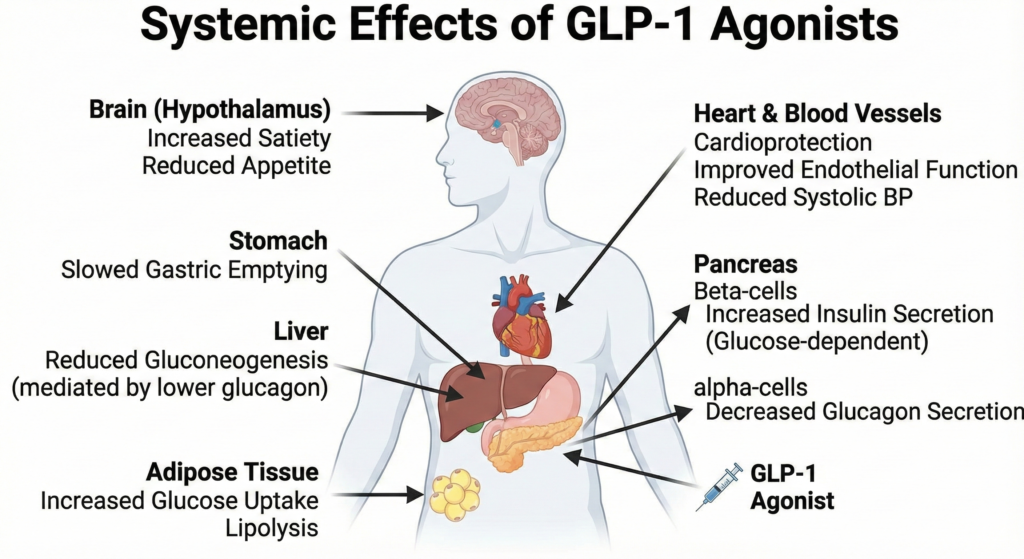
Figure 3. Systemic Effects of GLP-1 Agonists. Beyond the pancreas, these drugs act on the brain (satiety), stomach (delayed emptying), liver (reduced gluconeogenesis via lower glucagon), and heart (cardioprotection).
5. Individual Agents & Pharmacokinetics
5.1 Exenatide
- Source: Synthetic analog of Exendin-4 (found in saliva of the Gila monster lizard).
- Kinetics: Cleared renally (Avoid if GFR < 30 mL/min).
- Formulations:
- Byetta: Twice daily.
- Bydureon: Once weekly (microsphere technology).
5.2 Liraglutide
- Structure: Human GLP-1 analog with a C-16 fatty acid chain (palmitic acid) attached via a glutamate spacer.
- Mechanism of Prolongation: The fatty acid promotes binding to albumin, shielding the molecule from DPP-4 and slowing renal elimination.
- Uses: Approved for T2DM (Victoza) and high-dose for Obesity (Saxenda).
5.3 Semaglutide
- Structure: Human GLP-1 analog with a C-18 fatty acid diacid chain.
- Potency: More potent than Liraglutide with a longer half-life (~1 week).
- Oral Semaglutide (Rybelsus):
- The first oral peptide for diabetes.
- Technology: Co-formulated with SNAC (Sodium N-(8-[2-hydroxybenzoyl] amino) caprylate). SNAC locally increases stomach pH to prevent pepsin degradation and facilitates transcellular absorption across the gastric mucosa.
- Exam Note: Must be taken on an empty stomach with a sip of water, 30 mins before food.
5.4 Dulaglutide
- Structure: Two GLP-1 analogs covalently linked to an Fc fragment of human IgG4.
- Mechanism: Large size prevents renal filtration; Fc portion prevents degradation.
5.5 Tirzepatide (The “Twincretin”)
- Mechanism: A dual agonist of both GIP and GLP-1 receptors.
- Efficacy: Superior HbA1c reduction and weight loss compared to selective GLP-1 agonists (SURPASS trials).
- Note: GIP component enhances insulin secretion and may improve lipid handling.
6. Clinical Uses & Guidelines
- Type 2 Diabetes Mellitus:
- Not First Line: Metformin remains first-line.
- Guideline Placement (ADA/EASD): Preferred add-on therapy (over insulin) for patients with established ASCVD (Atherosclerotic Cardiovascular Disease) or high risk for it, regardless of HbA1c.
- Preferred for patients where weight loss is a priority.
- Preferred to minimize hypoglycemia.
- Obesity (Weight Management):
- Liraglutide (3.0 mg) and Semaglutide (2.4 mg) are approved for chronic weight management.
- Mechanism: Appetite suppression (reduces “food noise”) and delayed gastric emptying.
- Results: Semaglutide showed ~15% body weight loss in the STEP trials.
- Cardiovascular Risk Reduction:
- LEADER Trial (Liraglutide) and SUSTAIN-6 (Semaglutide) showed significant reduction in MACE (Major Adverse Cardiovascular Events: CV death, non-fatal MI, non-fatal stroke).
7. Adverse Effects
- Gastrointestinal (Most Common):
- Nausea, vomiting, diarrhea.
- Management: Dose-dependent. Start low and titrate slow. Usually transient (subsides after weeks).
- Pancreatitis:
- Slightly increased risk of acute pancreatitis. Patients should be counseled on symptoms (severe, persistent abdominal pain).
- Gallbladder Disease:
- Cholelithiasis and cholecystitis (likely due to rapid weight loss and reduced gallbladder motility).
- Thyroid C-Cell Tumors:
- Black Box Warning: Caused C-cell hyperplasia and Medullary Thyroid Carcinoma (MTC) in rodents. Human risk is unconfirmed but contraindications apply.
- Injection Site Reactions: Nodules (especially with Exenatide XR).
- Retinopathy Complications:
- Rapid improvement in glucose with Semaglutide was associated with temporary worsening of diabetic retinopathy (likely due to the rapidity of change, not direct toxicity).
8. Contraindications
- Personal or family history of Medullary Thyroid Carcinoma (MTC).
- Patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
- History of Pancreatitis (relative contraindication).
- Severe Gastroparesis (due to mechanism of delaying emptying).
9. Comparison: GLP-1 Agonists vs. DPP-4 Inhibitors
This comparison is a favorite topic for viva/board exams.
| Feature | GLP-1 Receptor Agonists | DPP-4 Inhibitors (Gliptins) |
| Examples | Liraglutide, Semaglutide | Sitagliptin, Linagliptin |
| Route | Subcutaneous (mostly) | Oral |
| Mechanism | Pharmacological levels of GLP-1 activity | Increases endogenous GLP-1 (physiologic levels) |
| HbA1c Reduction | High (1.0 – 1.8%) | Modest (0.5 – 0.8%) |
| Weight Effect | Significant Weight Loss | Weight Neutral |
| Gastric Emptying | Delayed (causes nausea) | No effect |
| CV Benefit | Proven Benefit (Lira, Sema, Dula) | Neutral (Saxagliptin risk of HF) |
| Side Effects | Nausea, Vomiting | Well tolerated (Rare joint pain) |
| Cost | High | Moderate |
10. References
- Brunton LL, Hilal-Dandan R, Knollmann BC. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics. 13th ed. New York: McGraw-Hill Education; 2018.
- Katzung BG. Basic & Clinical Pharmacology. 15th ed. New York: McGraw-Hill Education; 2021.
- American Diabetes Association. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes—2023. Diabetes Care. 2023;46(Supplement_1):S140-S157.
- Nauck MA, Quast DR, Wefers J, Meier JJ. GLP-1 receptor agonists in the treatment of type 2 diabetes – state-of-the-art. Mol Metab. 2021;46:101102.
- Ritter JM, Flower R, Henderson G, Loke YK, MacEwan D, Rang HP. Rang & Dale’s Pharmacology. 9th ed. Edinburgh: Elsevier; 2020.
Medical Disclaimer
The medical information on this post is for general educational purposes only and is provided by Pharmacology Mentor. While we strive to keep content current and accurate, Pharmacology Mentor makes no representations or warranties, express or implied, regarding the completeness, accuracy, reliability, suitability, or availability of the post, the website, or any information, products, services, or related graphics for any purpose. This content is not a substitute for professional medical advice, diagnosis, or treatment; always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition and never disregard or delay seeking professional advice because of something you have read here. Reliance on any information provided is solely at your own risk.