Introduction
Antiviral pharmacology has advanced from the single-agent era of idoxuridine to a modern arsenal including polymerase terminators, protease blockers, monoclonal antibodies, and host-directed entry inhibitors. Because viruses are obligate intracellular parasites, therapy emphasises selective toxicity—halting viral replication without irreparable host harm. This page delivers a section-wise, visually enhanced overview of the major antiviral classes, mechanisms, PK profiles, indications, resistance, and safety.
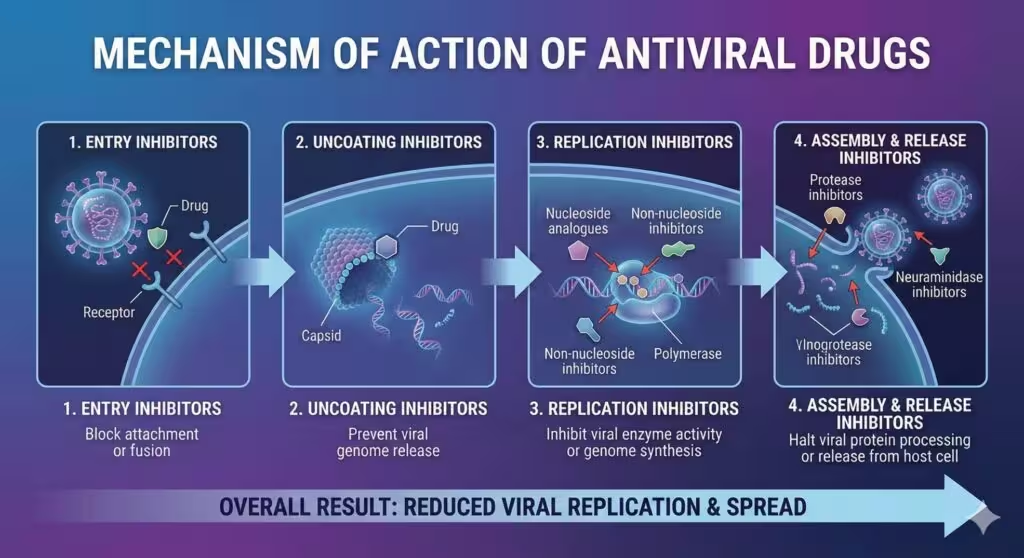
1. General Principles of Antiviral Therapy
- Virustatic: Most agents inhibit assembly/release rather than destroy virions.
- Stage specificity: Entry, uncoating, genome replication, integrase, or release.
- Early use: Greatest efficacy when given before peak viral load (e.g., oseltamivir ≤ 48 h).
- Combination therapy: Mandatory for HIV/HCV to curb resistance.
- Host factors: Renal/hepatic status, pregnancy, pharmacogenomics (CYP3A4, HLA-B*57:01) drive selection.
2. Anti-Herpesvirus Agents
2.1 Nucleoside Analogues
| Drug | Activation | Mechanism | PK Highlights | Major Toxicities |
|---|---|---|---|---|
| Acyclovir | HSV TK ➜ host kinases | DNA-pol chain termination | IV/PO; CSF ≈50 % | Crystalline nephropathy, neurotoxicity at high dose |
| Valacyclovir | Pro-acyclovir (↑ F 55 %) | Same | PO only | Similar, less frequent |
| Ganciclovir | UL97 kinase | DNA-pol inhibition + termination | IV; low PO F | Myelosuppression, CNS effects |
| Valganciclovir | Pro-ganciclovir | Same | PO F 60 % | As above |
| Cidofovir | Host kinases only | DNA-pol inhibitor | IV; long IC t½ | Renal toxicity (⇣ with probenecid + saline) |
| Foscarnet | None (pyrophosphate) | Blocks DNA-pol/RT | IV; CSF 70 % | Nephrotoxicity, ↓ Ca²⁺/Mg²⁺, seizures |
Resistance
TK-negative HSV mutants resist acyclovir; CMV UL97/DNA-pol mutants limit ganciclovir and cidofovir; foscarnet holds until polymerase mutations arise.
3. Anti-Influenza Drugs
3.1 M2 Ion-Channel Inhibitors
Amantadine & rimantadine block influenza A uncoating—now obsolete due to universal resistance.
3.2 Neuraminidase Inhibitors
| Drug | Spectrum | Route | Clinical Notes |
|---|---|---|---|
| Oseltamivir | A & B | PO | N/V; rare neuropsychiatric events |
| Zanamivir | A & B | Inhaled | Bronchospasm—avoid asthma/COPD |
| Peramivir | A & B | IV (single dose) | Diarrhoea |
3.3 Cap-Endonuclease Inhibitor
Baloxavir marboxil (single-dose PO) halts PA-mediated “cap-snatching”. I38T mutations reduce susceptibility.
4. Anti-RSV & Emerging Respiratory Viruses
- Ribavirin: Guanosine analogue (inhaled/PO/IV); haemolytic anaemia, teratogenic.
- Palivizumab / Nirsevimab: mAbs vs RSV F-protein for prophylaxis.
- Remdesivir: IV RdRp inhibitor (SARS-CoV-2); monitor LFTs.
- Molnupiravir: PO pro-mutagen; avoid pregnancy.
- Nirmatrelvir ± ritonavir: Oral 3CLpro inhibitor (Paxlovid); major CYP3A4 DDIs.
5. Anti-Hepatitis Agents
5.1 HBV
- Peg-IFN-α: Weekly SC; flu-like, depression.
- NRTI-like agents: Entecavir, TDF/TAF (high barrier) vs lamivudine (low-barrier M204V).
5.2 HCV – Direct-Acting Antivirals
| Class | Suffix | Prototype | Key Point |
|---|---|---|---|
| NS3/4A PI | -previr | Glecaprevir | CI in Child-Pugh B/C |
| NS5A Inhibitor | -asvir | Velpatasvir | Well-tolerated |
| NS5B Nuc Pol Inhib. | -buvir | Sofosbuvir | Bradycardia w/ amiodarone |
5.3 HDV
Bulevertide (SC) blocks NTCP entry; first licensed HDV drug.
6. Antiretroviral Therapy (HIV-1/2)
Standard regimen: 2 NRTIs + 1 high-barrier anchor (INSTI > boosted PI > NNRTI).
6.1 NRTIs
- Tenofovir (TDF/TAF): renal/BMD issues (less with TAF).
- Emtricitabine / Lamivudine: cytidine analogues (M184V).
- Abacavir: HLA-B*57:01 hypersensitivity.
- Zidovudine: anaemia; IV intrapartum prophylaxis.
6.2 NNRTIs & 6.3 Protease Inhibitors
Efavirenz (CNS dreams); rilpivirine (needs acid). Darunavir (rash, high barrier); atazanavir (hyperbilirubinaemia), all boosted by ritonavir/cobicistat (CYP3A4 DDIs).
6.4 INSTIs & 6.5 Entry/Fusion
Dolutegravir & bictegravir are first-line; long-acting IM cabotegravir/rilpivirine monthly. Maraviroc (CCR5), enfuvirtide (gp41), ibalizumab (post-attachment).
7. Anti-Poxvirus Drugs
- Tecovirimat: VP37 inhibitor (smallpox/monkeypox).
- Brincidofovir / Cidofovir: DNA-pol inhibitors for severe cases.
8. Pharmacokinetic Considerations
- Renal clearance: acyclovir, ganciclovir, TDF ➜ dose adjust CKD.
- Hepatic CYP3A4: PIs, NNRTIs, baloxavir ➜ beware DDIs.
- Prodrugs: valacyclovir, oseltamivir, baloxavir, TAF.
- Depot injections: cabotegravir/rilpivirine improve adherence but linger if ADRs occur.
9. Resistance Mechanisms
- HIV RT: M184V (lamivudine/emtricitabine) • K65R (tenofovir).
- HIV Protease: D30N, I50L.
- Influenza NA: H275Y (oseltamivir-R).
- HSV: TK-negative mutants.
10. Adverse-Effect Themes & Monitoring
- Bone marrow: ganciclovir, zidovudine ➜ CBC.
- Kidney: cidofovir, foscarnet, TDF ➜ Cr, phosphate.
- Neuropsychiatric: efavirenz dreams, IFN depression.
- Metabolic: lipodystrophy with older PIs.
- Mitochondrial: stavudine, didanosine.
11. Special Populations
- Pregnancy: Dolutegravir backbone; avoid ribavirin, molnupiravir.
- Neonates: IV acyclovir, oseltamivir, palivizumab prophylaxis.
- Transplant: Valganciclovir prophylaxis for CMV.
- Elderly: Polypharmacy ➜ PI/Paxlovid DDIs.
12. Immunomodulatory & Passive Agents
- Interferon-α: activates JAK-STAT antiviral genes; flu-like, depression.
- mAbs: RSV (palivizumab), SARS-CoV-2 (variant-dependent), HIV (ibalizumab).
- Vaccines reduce antiviral demand (influenza, HBV, SARS-CoV-2).
13. Emerging & Future Therapies
- Broad-spectrum RdRp inhibitors (favipiravir, bemnifosbuvir).
- Host-targeted cyclophilin inhibitors for HCV.
- CRISPR-Cas9 excision of HIV provirus (pre-clinical).
- Long-acting implants (islatravir, cabotegravir) for HIV PrEP.
- Universal coronavirus fusion blockers & nasal peptides.
14. Conclusion
The antiviral pharmacopeia epitomises the synergy of molecular virology and medicinal chemistry, converting lethal infections into manageable or curable diseases. Ongoing innovation remains essential to outpace resistance, PK variability, and emerging pathogens.
Selected Textbook References
- Goodman & Gilman’s Pharmacological Basis of Therapeutics (14th ed., 2023).
- Basic & Clinical Pharmacology (15th ed., 2021).
- Rang & Dale’s Pharmacology (10th ed., 2022).
- Mandell, Douglas, Bennett’s Infectious Diseases (9th ed., 2020).
- De Clercq E., Li G. Approved Antiviral Drugs (2021).
- Antiviral Chemotherapy & Chemoprophylaxis (4th ed., 2019).
- Hill A. Global Antiviral Pharmacology (2022).
Medical Disclaimer
The medical information on this post is for general educational purposes only and is provided by Pharmacology Mentor. While we strive to keep content current and accurate, Pharmacology Mentor makes no representations or warranties, express or implied, regarding the completeness, accuracy, reliability, suitability, or availability of the post, the website, or any information, products, services, or related graphics for any purpose. This content is not a substitute for professional medical advice, diagnosis, or treatment; always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition and never disregard or delay seeking professional advice because of something you have read here. Reliance on any information provided is solely at your own risk.

