📋 Table of Contents
- Introduction to Anticoagulants
- The Coagulation Cascade – A Quick Review
- Classification of Anticoagulants
- Unfractionated Heparin (UFH)
- Low Molecular Weight Heparins (LMWH)
- Warfarin & Coumarin Derivatives
- Direct Oral Anticoagulants (DOACs)
- Other Anticoagulants
- Comparative Analysis
- Monitoring & Reversal
- Clinical Scenarios & Guidelines
- Drug Interactions
- Special Populations
- Key Takeaways
1. Introduction to Anticoagulants
Anticoagulants are a class of drugs that prevent or reduce the formation of blood clots (thrombi) by interfering with the coagulation cascade. They are among the most commonly prescribed medications worldwide, and their appropriate use can be life-saving — while their misuse can lead to devastating hemorrhagic complications.
🔑 Key Distinction
Anticoagulants prevent clot formation by inhibiting coagulation factors. They are different from antiplatelets (which inhibit platelet aggregation) and fibrinolytics/thrombolytics (which dissolve existing clots). Understanding this distinction is critical for choosing appropriate therapy.
The history of anticoagulant therapy dates back to the early 20th century. Heparin was discovered in 1916 by Jay McLean, while warfarin’s discovery came from investigating a hemorrhagic disease in cattle that consumed spoiled sweet clover. Today, we have an expanding arsenal of anticoagulants, including the revolutionary Direct Oral Anticoagulants (DOACs) that have transformed clinical practice.
📊 Why Are Anticoagulants Important?
- Venous thromboembolism (VTE) affects ~1-2 per 1000 adults annually
- Atrial fibrillation affects 33+ million people globally
- Pulmonary embolism is a leading cause of preventable hospital death
- Mechanical heart valves require lifelong anticoagulation
⚠️ The Balancing Act
Anticoagulant therapy is a delicate balance between preventing thrombosis and avoiding hemorrhage. Too little anticoagulation risks clot formation; too much risks dangerous bleeding. This is why understanding pharmacology, monitoring, and patient factors is essential.
2. The Coagulation Cascade – A Quick Review
To understand how anticoagulants work, we must first review the coagulation cascade. This complex series of enzymatic reactions ultimately converts soluble fibrinogen into insoluble fibrin, forming the structural framework of a blood clot.
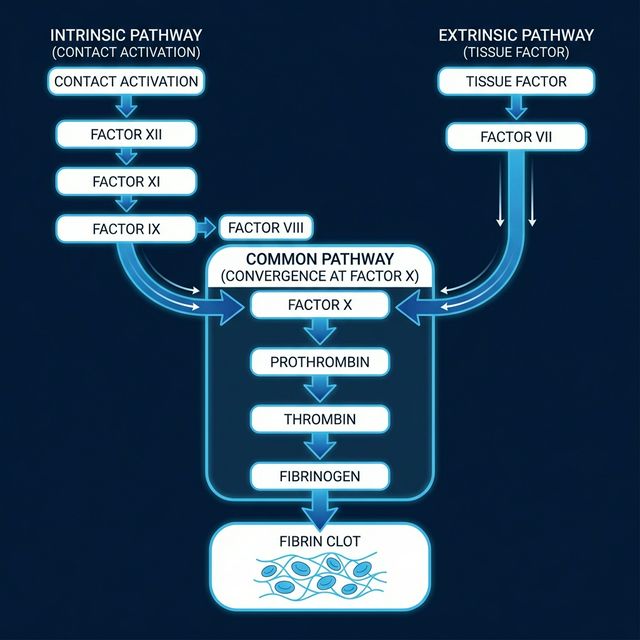
Figure 1: The Coagulation Cascade — Intrinsic and Extrinsic pathways converging at the Common Pathway
The Two Pathways
Intrinsic Pathway (Contact Activation)
Triggered by exposure to subendothelial collagen. Involves factors XII → XI → IX → VIII. Measured by aPTT (activated Partial Thromboplastin Time).
Extrinsic Pathway (Tissue Factor)
Triggered by tissue factor (TF) released from damaged tissue. Involves factor VII. Measured by PT/INR (Prothrombin Time / International Normalized Ratio).
🔄 The Common Pathway
Both pathways converge at Factor X activation. Factor Xa, along with Factor Va, converts prothrombin (Factor II) → thrombin (Factor IIa). Thrombin then converts fibrinogen → fibrin, which is cross-linked by Factor XIII to form a stable clot.
Clinical Pearl: Vitamin K-Dependent Factors
Factors II, VII, IX, and X (mnemonic: “1972” — the year of the classic reference, or remember 2, 7, 9, 10) require Vitamin K for their synthesis in the liver. Additionally, Protein C and Protein S (natural anticoagulants) are also Vitamin K-dependent. This is the basis of warfarin’s mechanism of action.
3. Classification of Anticoagulants
| Category | Drugs | Route | Primary Target |
|---|---|---|---|
| Unfractionated Heparin (UFH) | Heparin sodium | IV / SC | Antithrombin III → inhibits IIa, Xa, IXa, XIa, XIIa |
| Low Molecular Weight Heparin (LMWH) | Enoxaparin, Dalteparin, Tinzaparin, Nadroparin | SC | Antithrombin III → primarily inhibits Factor Xa |
| Vitamin K Antagonists (VKA) | Warfarin, Acenocoumarol, Phenprocoumon | Oral | Inhibits Vitamin K epoxide reductase (VKORC1) |
| Direct Thrombin Inhibitors (DTI) | Dabigatran (oral), Bivalirudin, Argatroban (parenteral) | Oral IV | Direct inhibition of thrombin (Factor IIa) |
| Direct Factor Xa Inhibitors | Rivaroxaban, Apixaban, Edoxaban, Betrixaban | Oral | Direct inhibition of Factor Xa |
| Indirect Factor Xa Inhibitors | Fondaparinux | SC | Antithrombin III → selective Factor Xa inhibition |
4. Unfractionated Heparin (UFH)
Heparin is a naturally occurring, highly sulfated glycosaminoglycan found primarily in mast cells. It is one of the oldest and most widely used anticoagulants, particularly in acute clinical settings.
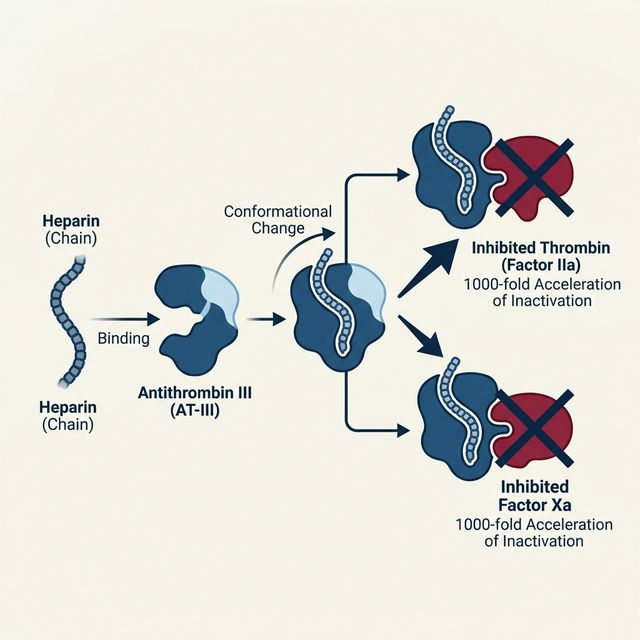
Figure 2: Heparin’s Mechanism of Action — Binding to Antithrombin III accelerates factor inactivation by 1000-fold
⚙️ Mechanism of Action
Heparin binds to Antithrombin III (AT-III) via a specific pentasaccharide sequence, causing a conformational change that accelerates AT-III’s ability to inactivate coagulation factors by 1000-fold. The heparin-AT-III complex primarily inhibits:
- Thrombin (Factor IIa) — requires heparin chain ≥ 18 saccharide units to bridge thrombin and AT-III
- Factor Xa — requires only the pentasaccharide sequence
- Also inhibits Factors IXa, XIa, and XIIa
📋 Pharmacokinetics
- Route: IV (preferred) or deep SC
- NOT given IM (risk of hematoma)
- Onset: Immediate (IV), 1-2 hrs (SC)
- Half-life: 1-2 hours (dose-dependent)
- Metabolism: Hepatic (heparinase) + RES
- Does NOT cross placenta — safe in pregnancy
- NOT absorbed orally (large, charged molecule)
🏥 Clinical Uses
- Acute DVT and Pulmonary Embolism
- Acute coronary syndromes (ACS)
- During and after cardiac/vascular surgery
- Cardiopulmonary bypass
- Hemodialysis (to prevent clotting in circuit)
- Bridge therapy for warfarin initiation
- DIC (low dose)
- Pregnancy-associated thrombosis
⚠️ Heparin-Induced Thrombocytopenia (HIT)
HIT is the most feared complication of heparin therapy. There are two types:
- HIT Type I (non-immune): Mild, transient ↓ platelets (rarely < 100,000). Occurs within first 2 days. Benign — continue heparin.
- HIT Type II (immune-mediated): Severe ↓ platelets (>50% drop or < 100,000). Occurs 5-14 days after starting heparin. Caused by IgG antibodies against heparin-PF4 (platelet factor 4) complex. Paradoxically causes thrombosis, not bleeding!
Management of HIT II: Immediately stop ALL heparin products. Use alternative anticoagulants: Argatroban (hepatic metabolism — preferred in renal impairment) or Bivalirudin. Never give warfarin alone during acute HIT (risk of warfarin-induced skin necrosis).
Clinical Pearl: Protamine Reversal
Protamine sulfate is the antidote for heparin overdose. It is a positively charged protein that binds to negatively charged heparin. 1 mg protamine neutralizes ~100 units of heparin. Only give the amount expected to be circulating (based on half-life). Caution: protamine can cause hypotension, anaphylaxis (especially in patients with prior protamine exposure, fish allergy, or vasectomy), and has its own mild anticoagulant effect in excess.
5. Low Molecular Weight Heparins (LMWH)
LMWHs are derived from UFH by chemical or enzymatic depolymerization, producing fragments approximately one-third the size of unfractionated heparin (average MW: 4000-5000 Da vs 15,000 Da for UFH).
⚙️ Mechanism of Action
Like UFH, LMWHs work through AT-III. However, their shorter chain length means they primarily inhibit Factor Xa (anti-Xa:anti-IIa ratio of ~3:1 for enoxaparin, up to 4:1 for others) rather than thrombin. They require the same pentasaccharide sequence to bind AT-III but are too short to bridge AT-III to thrombin effectively.
| Feature | UFH | LMWH |
|---|---|---|
| Molecular weight | 12,000-15,000 Da | 4,000-5,000 Da |
| Anti-Xa:Anti-IIa | 1:1 | 2:1 to 4:1 |
| Bioavailability (SC) | ~30% | ~90% |
| Half-life | 1-2 hours | 4-6 hours |
| Monitoring | aPTT required | Usually not needed (anti-Xa if required) |
| Dose response | Variable | Predictable |
| HIT risk | Higher (~3%) | Lower (~0.2%) |
| Protamine reversal | 100% effective | ~60% effective |
| Renal clearance | No | Yes — caution in renal impairment |
✅ Advantages of LMWH over UFH
Better bioavailability, predictable dose-response (fixed-dose regimen based on weight), longer half-life (once or twice daily dosing), lower HIT risk, can be self-administered at home (outpatient DVT treatment), and usually no lab monitoring needed.
Examples & Doses
- Enoxaparin: 1 mg/kg SC q12h (treatment) or 40 mg SC daily (prophylaxis)
- Dalteparin: 200 IU/kg SC daily (treatment)
- Tinzaparin: 175 IU/kg SC daily
⚠️ Cautions
- Renal impairment (CrCl <30): Dose reduction or use UFH instead
- Obesity: Use actual body weight for dosing
- Pregnancy: Safe — does not cross placenta
- Spinal/epidural: Risk of epidural hematoma — follow timing guidelines
6. Warfarin & Coumarin Derivatives
Warfarin (Coumadin®) remains the most widely prescribed oral anticoagulant worldwide, despite the introduction of DOACs. It is the gold standard for certain indications and the only oral anticoagulant approved for mechanical heart valves.
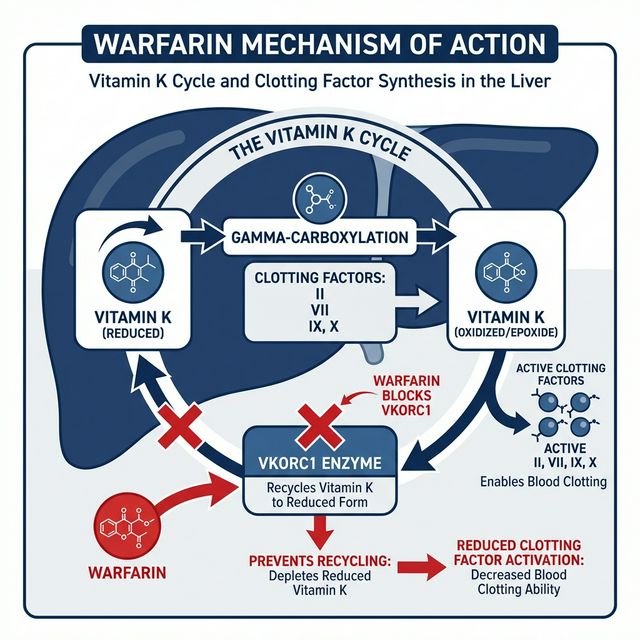
Figure 3: Warfarin’s Mechanism of Action — Blocking VKORC1 prevents recycling of Vitamin K, inhibiting synthesis of factors II, VII, IX, X
⚙️ Mechanism of Action
Warfarin inhibits Vitamin K Epoxide Reductase (VKORC1), the enzyme that recycles oxidized vitamin K back to its active (reduced) form. Without reduced vitamin K, the liver cannot perform gamma-carboxylation of glutamic acid residues on factors II, VII, IX, and X, rendering them non-functional (PIVKA — Proteins Induced by Vitamin K Absence).
Warfarin blocks the recycling step, depleting reduced vitamin K over time. Effect is delayed because existing functional factors must be cleared first.
Clinical Pearl: Delayed Onset & “Warfarin Paradox”
Warfarin’s anticoagulant effect takes 3-5 days to develop fully because it depends on the clearance of existing functional clotting factors (Factor VII has the shortest half-life at ~6 hours, but Factor II has a half-life of ~60 hours). During the initial period, Protein C (half-life ~8 hours) is depleted faster than the procoagulant factors, creating a transient hypercoagulable state. This is why heparin bridging is essential when starting warfarin.
📋 Pharmacokinetics
- Route: Oral (>95% bioavailability)
- Onset: 8-12 hours; full effect in 3-5 days
- Duration: 2-5 days after discontinuation
- Protein binding: ~99% (albumin)
- Metabolism: CYP2C9 (major), CYP3A4
- Half-life: 36-42 hours (R-warfarin longer)
- Crosses placenta: ❌ TERATOGENIC — absolutely contraindicated in 1st trimester
🎯 Target INR Values
- DVT/PE treatment: INR 2.0-3.0
- Atrial fibrillation: INR 2.0-3.0
- Mechanical aortic valve: INR 2.0-3.0
- Mechanical mitral valve: INR 2.5-3.5
- Recurrent VTE on warfarin: INR 2.5-3.5
⚠️ Warfarin Side Effects & Complications
- Bleeding — most common adverse effect (GI, intracranial, hematuria)
- Warfarin-induced skin necrosis — occurs 3-8 days after initiation; due to rapid depletion of Protein C in patients with Protein C deficiency; presents as painful, purplish skin lesions progressing to necrosis
- Purple toe syndrome — cholesterol microembolization 3-8 weeks after starting warfarin
- Teratogenicity — nasal hypoplasia, stippled epiphyses (1st trimester); CNS abnormalities (any trimester)
🔄 Warfarin Reversal
- Mild (INR 5-9, no bleeding): Hold warfarin, ± low-dose Vitamin K (1-2.5 mg oral)
- Significant INR elevation (INR >9, no bleeding): Vitamin K 2.5-5 mg oral
- Serious/life-threatening bleeding: IV Vitamin K 10 mg + 4-Factor Prothrombin Complex Concentrate (4F-PCC) or FFP
- Vitamin K takes 12-24 hours to work (requires synthesis of new factors)
- 4F-PCC provides immediate reversal by supplying functional factors directly
7. Direct Oral Anticoagulants (DOACs)
DOACs (also called NOACs — Novel Oral Anticoagulants, though “direct” is now preferred) represent a paradigm shift in anticoagulant therapy. They directly inhibit specific coagulation factors without requiring AT-III as a cofactor, offer predictable pharmacokinetics, and generally do not require routine monitoring.
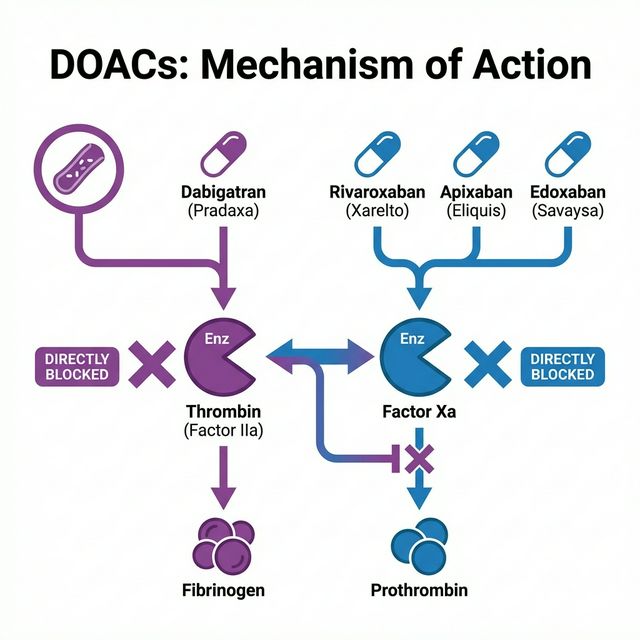
Figure 4: DOACs Mechanism of Action — Dabigatran directly inhibits Thrombin (IIa), while Rivaroxaban, Apixaban, and Edoxaban directly inhibit Factor Xa
| Drug | Target | Bioavailability | Half-life | Renal Excretion | Specific Antidote |
|---|---|---|---|---|---|
| Dabigatran (Pradaxa®) | Direct Thrombin (IIa) Inhibitor | 6-7% | 12-17 hrs | 80% | Idarucizumab |
| Rivaroxaban (Xarelto®) | Direct Factor Xa Inhibitor | 80-100% (with food) | 5-13 hrs | 33% | Andexanet alfa |
| Apixaban (Eliquis®) | Direct Factor Xa Inhibitor | 50% | 8-15 hrs | 27% | Andexanet alfa |
| Edoxaban (Savaysa®) | Direct Factor Xa Inhibitor | 62% | 10-14 hrs | 50% | Andexanet alfa |
✅ DOAC Advantages
- Fixed dosing — no routine monitoring
- Rapid onset (1-4 hours)
- Shorter half-life → faster offset
- Fewer drug/food interactions vs warfarin
- Lower intracranial hemorrhage risk
- No heparin-bridging needed
- Specific reversal agents available
❌ DOAC Limitations
- Higher cost than warfarin
- NOT for mechanical heart valves (RE-ALIGN trial — dabigatran caused more thromboembolism and bleeding!)
- Renal dose adjustment required
- GI bleeding risk may be higher (dabigatran, rivaroxaban)
- No reliable routine monitoring test
- Compliance critical (short half-life means missed doses = risk)
🔬 Dabigatran – Special Considerations
Dabigatran is administered as the prodrug dabigatran etexilate and is the only DOAC that is a direct thrombin inhibitor. It must be stored in its original packaging (hygroscopic). It has the highest renal dependence (80%), making it the most problematic in renal failure. Its specific antidote Idarucizumab (Praxbind®) is a humanized monoclonal antibody fragment that binds dabigatran with 350x higher affinity than thrombin. Dabigatran is the only DOAC that can be partially removed by hemodialysis.
8. Other Anticoagulants
Fondaparinux (Arixtra®)
A synthetic pentasaccharide that selectively binds AT-III to inhibit Factor Xa only. Unlike heparin, it does not inhibit thrombin and has zero risk of HIT (does not bind PF4). Given SC once daily. Used for DVT prophylaxis/treatment and as an alternative in HIT. Renal excretion — contraindicated if CrCl <30. No antidote (protamine not effective; recombinant Factor VIIa may be used in emergencies).
Bivalirudin (Angiomax®)
A synthetic 20-amino acid peptide that is a direct thrombin inhibitor. Used primarily in percutaneous coronary intervention (PCI). Short half-life (25 min). Advantage: partially cleared by enzymatic cleavage (not only renal). Thrombin slowly cleaves the bivalirudin molecule, allowing temporary inhibition.
Argatroban
A synthetic direct thrombin inhibitor given by continuous IV infusion. Preferred in HIT with renal failure because it is hepatically metabolized. Half-life: 39-51 minutes. Monitored by aPTT. Important: falsely elevates INR when transitioning to warfarin.
Danaparoid (Orgaran®) — Discontinued in many markets
A heparinoid mixture (heparan sulfate, dermatan sulfate, chondroitin sulfate) with primarily anti-Xa activity. Was used as an alternative in HIT, but has ~10% cross-reactivity with HIT antibodies.
9. Comparative Analysis
| Feature | Warfarin | Dabigatran | Rivaroxaban | Apixaban |
|---|---|---|---|---|
| Target | VKORC1 | Thrombin (IIa) | Factor Xa | Factor Xa |
| Onset | 3-5 days | 1-2 hrs | 2-4 hrs | 3-4 hrs |
| Half-life | 36-42 hrs | 12-17 hrs | 5-13 hrs | 8-15 hrs |
| Monitoring | INR (essential) | Not routine | Not routine | Not routine |
| Food interactions | Vitamin K-rich foods | None significant | Take with food | None significant |
| Renal concern | Minimal | High (80%) | Moderate (33%) | Low (27%) |
| Antidote | Vitamin K + 4F-PCC | Idarucizumab | Andexanet alfa | Andexanet alfa |
| Mech. valve | ✅ Yes | ❌ No | ❌ No | ❌ No |
| Pregnancy | ❌ Teratogenic | ❌ No data | ❌ No data | ❌ No data |
| Cost | Low | High | High | High |
10. Monitoring & Reversal Agents
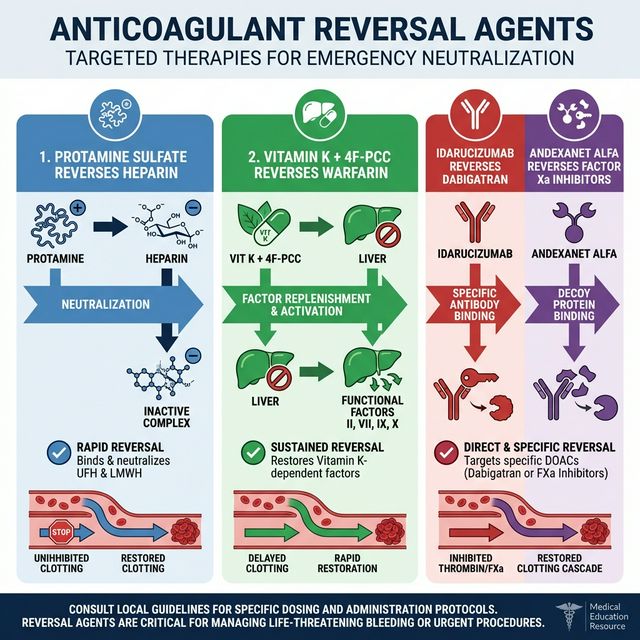
Figure 5: Anticoagulant Reversal Agents — Targeted therapies for emergency neutralization of each anticoagulant class
| Anticoagulant | Monitoring Test | Reversal Agent |
|---|---|---|
| UFH | aPTT (target: 1.5-2.5x control) or anti-Xa level | Protamine sulfate (1 mg per 100 units heparin) |
| LMWH | Anti-Xa level (if needed: obesity, renal impairment, pregnancy) | Protamine (~60% reversal) |
| Warfarin | PT/INR | Vitamin K + 4F-PCC or FFP |
| Dabigatran | dTT (dilute thrombin time), Ecarin clotting time | Idarucizumab (Praxbind®) |
| Rivaroxaban/Apixaban | Drug-specific anti-Xa assay | Andexanet alfa (Andexxa®) or 4F-PCC |
| Fondaparinux | Anti-Xa level | No specific antidote; rFVIIa in emergencies |
11. Clinical Scenarios & Guideline Recommendations
🫀 Atrial Fibrillation (AF)
Use CHA₂DS₂-VASc score to assess stroke risk. Score ≥2 (men) or ≥3 (women) → anticoagulate. DOACs are preferred over warfarin for non-valvular AF (AHA/ACC/HRS guidelines). Warfarin required for valvular AF (moderate-severe mitral stenosis or mechanical valve).
🦵 VTE (DVT/PE) Treatment
Initial: LMWH or DOAC. Long-term (≥3 months): DOAC preferred or warfarin (INR 2-3). Extended therapy for unprovoked VTE or recurrent events. Cancer-associated VTE: LMWH or DOAC (edoxaban, rivaroxaban) preferred over warfarin.
🏨 VTE Prophylaxis
Surgical patients: LMWH (enoxaparin 40 mg SC daily) or UFH 5000 U SC q8-12h. Medical patients (Padua score ≥4): pharmacologic prophylaxis. Orthopedic surgery: LMWH, rivaroxaban, apixaban, or warfarin for 10-35 days.
❤️ Acute Coronary Syndrome
UFH remains standard adjunctive therapy with antiplatelet agents. LMWH (enoxaparin) is an alternative. Fondaparinux may be used in NSTEMI. Bivalirudin for PCI. After stent placement, dual antiplatelet therapy ± short-course anticoagulation as needed.
12. Drug Interactions
Warfarin Interactions (Most Clinically Significant)
Warfarin has one of the longest drug interaction lists of any medication. Key interactions:
| Increase Warfarin Effect (↑ INR) | Decrease Warfarin Effect (↓ INR) |
|---|---|
| Amiodarone Fluconazole Metronidazole TMP-SMX Ciprofloxacin Omeprazole NSAIDs Cranberry juice Acetaminophen (chronic) | Rifampin Carbamazepine Phenytoin Phenobarbital St. John’s Wort Vitamin K-rich foods Cholestyramine |
Clinical Pearl: DOAC Interactions
DOACs have fewer interactions but are affected by P-glycoprotein (P-gp) and CYP3A4 inhibitors/inducers. Strong dual P-gp/CYP3A4 inhibitors (ketoconazole, ritonavir) are contraindicated with rivaroxaban and apixaban. Strong P-gp inducers (rifampin) reduce all DOAC levels significantly. Dabigatran is most affected by P-gp modulators since it is not CYP metabolized.
13. Special Populations
🤰 Pregnancy
- LMWH or UFH are the agents of choice
- Warfarin: Absolutely contraindicated in 1st trimester (teratogenic — warfarin embryopathy). May be used cautiously in 2nd trimester for high-risk mechanical valves
- DOACs: Contraindicated — insufficient safety data
- Fondaparinux: Limited data, used only if heparin allergy/HIT
🧓 Elderly
- Increased bleeding risk — assess with HAS-BLED score
- Apixaban may be preferred DOAC (lowest renal dependence, lower GI bleed risk)
- Warfarin: start with lower doses (2.5-5 mg)
- Fall risk assessment is important but falls alone are NOT a contraindication
🏥 Renal Impairment
- CrCl <30: Avoid dabigatran, edoxaban; reduce apixaban dose; avoid LMWH or reduce dose
- CrCl <15 or dialysis: UFH or warfarin preferred. Apixaban has some data supporting use in dialysis
- Monitor anti-Xa levels for LMWH in renal impairment
🏥 Hepatic Impairment
- Already impaired coagulation factor synthesis
- Warfarin: highly variable response — use with extreme caution
- DOACs: avoid in severe hepatic impairment (Child-Pugh C); rivaroxaban additionally avoided in Child-Pugh B
- UFH: may be preferred but monitoring is unreliable
14. Key Takeaways
📚 Recommended Resources for Further Reading
- ACCP (CHEST) Guidelines for Antithrombotic Therapy
- AHA/ACC/HRS Guidelines for Atrial Fibrillation Management
- ASH Guidelines for VTE
- Goodman & Gilman’s Pharmacological Basis of Therapeutics
- Katzung Basic & Clinical Pharmacology
📋 Disclaimer: This article is intended for educational purposes only and should not be used as a substitute for professional medical advice, diagnosis, or treatment. Always consult qualified healthcare professionals for clinical decision-making.
Medical Disclaimer
The medical information on this post is for general educational purposes only and is provided by Pharmacology Mentor. While we strive to keep content current and accurate, Pharmacology Mentor makes no representations or warranties, express or implied, regarding the completeness, accuracy, reliability, suitability, or availability of the post, the website, or any information, products, services, or related graphics for any purpose. This content is not a substitute for professional medical advice, diagnosis, or treatment; always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition and never disregard or delay seeking professional advice because of something you have read here. Reliance on any information provided is solely at your own risk.