Introduction
Linzagolix, brand named Yselty®, is a selective, orally administered, non-peptide small molecule gonadotrophin-releasing hormone (GnRH) receptor antagonist developed by Kissei Pharmaceutical, aimed at treating uterine fibroids and endometriosis in women of reproductive age1. Here is an in-depth insight into Linzagolix’s pharmacology, along with some recent updates:
Mechanism of Action
Linzagolix operates by binding to and blocking the GnRH receptor located in the pituitary gland. This interaction modulates the hypothalamic-pituitary-gonadal axis, leading to a dose-dependent reduction in the serum levels of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), as well as serum estradiol levels1.
Therapeutic Uses
Uterine Fibroids: Linzagolix has been approved in the European Union for treating moderate to severe symptoms of uterine fibroids in adult women of reproductive age. This approval extends the treatment options available for managing this condition, offering different treatment regimens, including short-term use to reduce uterine and fibroid volumes2.
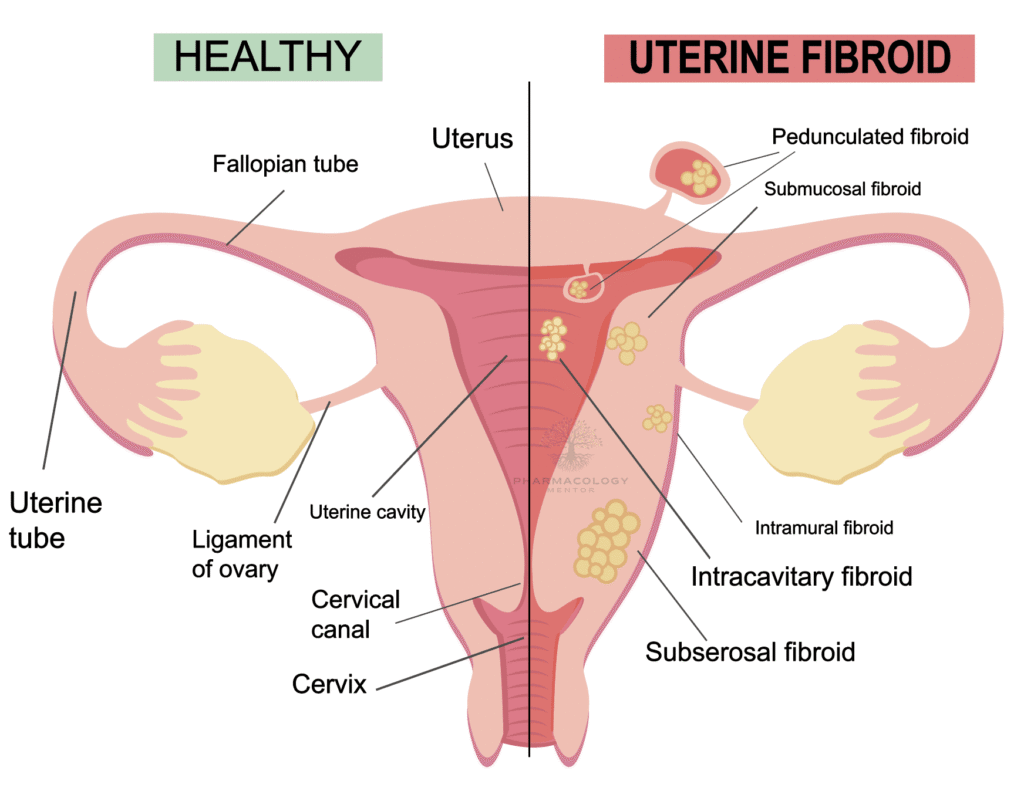
Endometriosis-Associated Pain: Linzagolix is in phase 3 clinical development for managing pain associated with endometriosis, showcasing its potential in broadening the therapeutic options for this debilitating condition1.
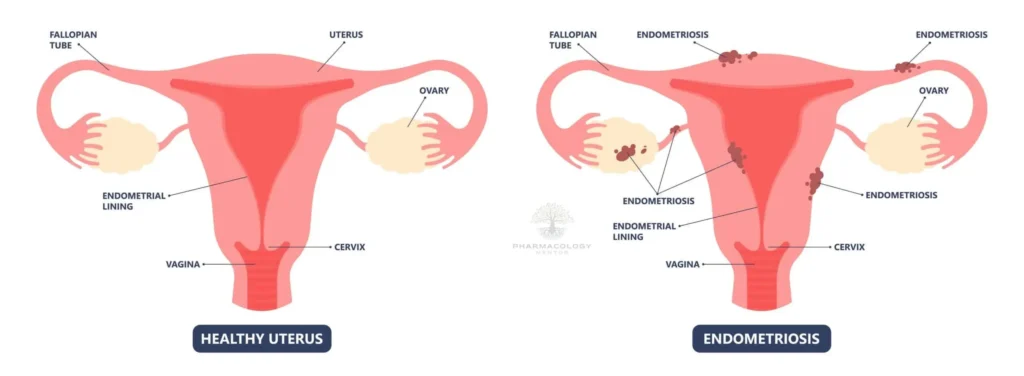
Recent Updates
- Approval: As of June 2022, Linzagolix received approval in the EU for the treatment of moderate to severe symptoms of uterine fibroids in adult women of reproductive age. Additionally, it is under regulatory review in the USA for the same indication1.
- Clinical Trials: Recent clinical trials, including a randomized, double-blind, placebo-controlled trial, investigated the long-term efficacy and safety of Linzagolix in 484 women with moderate to severe Endometriosis-Associated Pain (EAP). The trial evaluated two dose regimens: Linzagolix 200 mg once daily in combination with hormonal add-back therapy (ABT) and Linzagolix 75 mg without ABT3.
- Reduced Menstrual Bleeding: Recent studies found that Linzagolix (100 mg or 200 mg) with or without add-back therapy significantly reduced heavy menstrual bleeding, showcasing its potential benefits in managing symptoms associated with uterine leiomyomas4.
Conclusion
Linzagolix’s development, clinical trials, and recent approval in the EU reflect the ongoing efforts to provide better therapeutic solutions for women suffering from uterine fibroids and endometriosis. Its mechanism of action, which centrally modulates the hormonal axis, holds promise in offering a valuable treatment option for these prevalent gynecological conditions.
📚 AI Pharma Quiz Generator
🎉 Quiz Results
Medical Disclaimer
The medical information on this post is for general educational purposes only and is provided by Pharmacology Mentor. While we strive to keep content current and accurate, Pharmacology Mentor makes no representations or warranties, express or implied, regarding the completeness, accuracy, reliability, suitability, or availability of the post, the website, or any information, products, services, or related graphics for any purpose. This content is not a substitute for professional medical advice, diagnosis, or treatment; always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition and never disregard or delay seeking professional advice because of something you have read here. Reliance on any information provided is solely at your own risk.



